Research Focus |
|
尿源性膀胱癌类器官作为肿瘤纵向反应监测和治疗适应性的工具 2024-04-25 08:42:45 浏览次数:2978 | |
| 尿源性膀胱癌类器官作为肿瘤纵向反应监测和治疗适应性的工具 来源:仪方生物 www.yeslab.com 背景 膀胱癌是世界上最常见的癌症类型之一。一般来说,研究依赖于侵入性抽样策略。 方法: 在这里,我们直接从尿液中产生膀胱癌类器官。在本研究中,我们从22例非肌层及肌层浸润性膀胱肿瘤患者中建立了12株类器官细胞系,有效率为55%。 结果 尿类肿瘤的组织病理学特征与原发性膀胱肿瘤相似。从遗传学角度看,类器官与原发肿瘤的单核苷酸多态性(92.56%)和插入与缺失(91.54%)高度一致。此外,这些尿酸类化合物对膀胱癌药物表现出敏感性,类似于其组织来源的类有机物。对一例接受全身免疫治疗的患者进行纵向生成的类肿瘤和类尿酸的基因分析,确定可能指导二线治疗选择的改变。成功的治疗适应随后证明在尿酸设置。 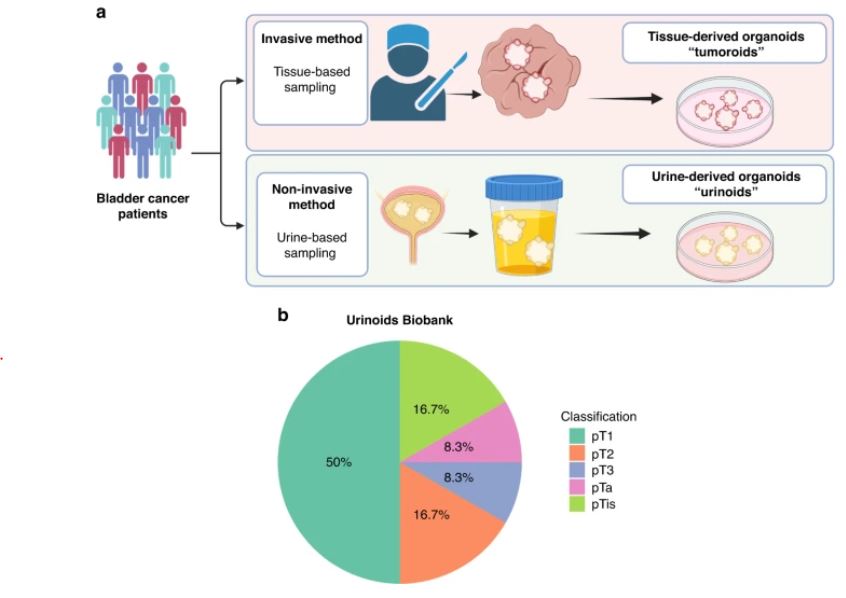 结论: 因此,膀胱癌类器官作为一种无创的肿瘤发病机制、纵向药物反应监测和治疗适应性的平台,可以促进膀胱癌精准医学的发展。 膀胱癌分别位列全球男性和女性最常见的五大癌症和十大癌症之列[1]。2022年,全世界有573000多名患者被诊断为膀胱癌(IARC)。尿路上皮癌(UCC)是膀胱癌(BC)的主要组织病理学亚型,在初始分期时表现为非肌层浸润性膀胱癌(NMIBC;73%)或肌层浸润性膀胱癌(MIBC)[2,3,4]。一般来说,NMIBC复发率高(高达84%),但很少转移,而MIBC是一种侵袭性疾病,复发和转移风险高(高达50%)[3,4]。目前以顺铂为基础的新辅助联合化疗方案在大约25%的病例中导致完全的病理反应[5]。因此,需要评估肿瘤生物学和评估单个膀胱肿瘤的化疗敏感性,以指导个体化膀胱癌治疗[6,7,8,9,10,11]。膀胱癌的大规模遗传分析已经确定了驱动因素,如TP53、ARID1A、PIK3CA、FGFR3、STAG2和ERBB2,并产生了几种分子分类方法[12],其中有一致的标记物,如TP63(过渡/中间细胞)、KRT5(基本类)、KRT20(管腔类)和UP3A(尿路上皮分化)[11、13、14、15、16]。然而,在临床实践中应用这些信息来指导治疗决策仍然具有挑战性[17]。此外,药物治疗本身可能导致肿瘤特征的改变,如遗传不稳定性,并导致特定分子亚克隆的富集,这可能导致药物敏感性和获得性耐药性的改变[17,18,19,20]。因此,允许纵向监测药物反应的平台在制定个性化和适应性治疗策略方面具有重大价值。 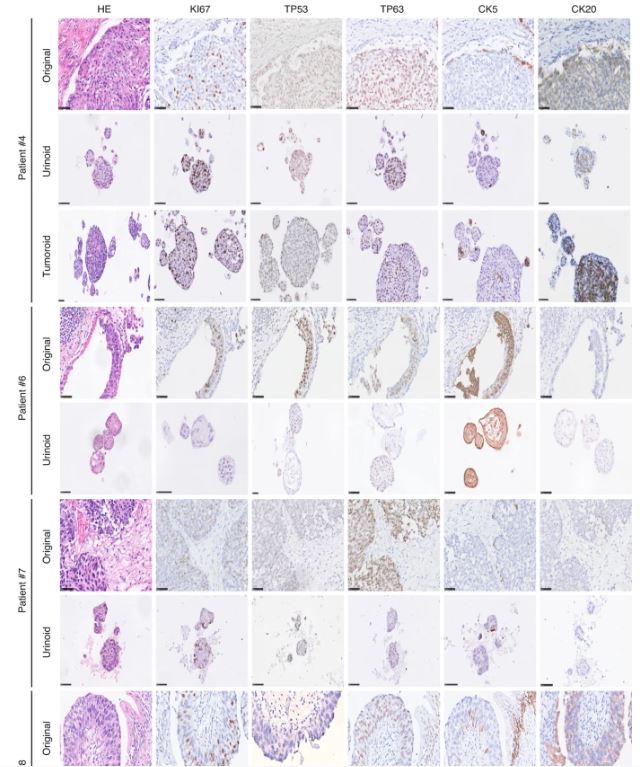 尿液作为膀胱癌类器官无创生成的来源 来自乌得勒支大学医学中心的膀胱癌患者被邀请参加这一前瞻性的概念验证项目,其方式是获得治疗泌尿科医生或执业护士的知情同意。肿瘤组织活检和尿液样本取自同意接受经尿道膀胱肿瘤切除术(TUR-BT)或根治性膀胱切除术的膀胱癌患者。使用经尿道导管收集尿液样本(图1)。随后将尿液来源的细胞培养在膀胱癌类器官培养基中以产生类器官(类尿苷)[16]。同时,从切除的肿瘤组织(tumoroids)中生成膀胱癌类器官,这些组织也被新鲜冷冻以进行组织病理学和遗传分析。本研究共纳入22例膀胱癌患者,建立了12个类器官细胞系的活体生物库(建立效率55%;图1b,表1和补充表1)。尿类物质来源于不同疾病分期的患者,包括NMIBC(Ta(8.3%);CIS(16.7%);T1(50%)和MIBC(T2(16.7%);T3(8.3%)(图1b,表1和补充表1)。  尿酸类培养物捕获原始肿瘤组织的组织病理学特征 通过苏木精-伊红(HE)染色和免疫组织化学(IHC)检测膀胱癌亚型特异性和增殖标记物的表达(图2和补充图1a-c),分析所有尿样、成对肿瘤样和原始非培养肿瘤组织。HE染色显示原始肿瘤组织的组织病理学形态在各自的瘤样和尿样培养物中重现(图2和补充图1c)。病理学家对Ki67进行的IHC分析确定,在原始肿瘤组织和成对的瘤样和尿样中,阳性(增殖)肿瘤细胞的百分比非常相似(图2)。此外,p53、p63、CK5和CK20的表达在原始肿瘤组织和成对的瘤样和尿样中高度一致(图2和补充表2)。所有原始组织、瘤样组织和尿样组织的核肾标记物PAX8均为阴性(补充图2)。所有肿瘤组织和类器官系的尿路上皮分化标记物uroplakin 3a(UP3A)(补充图2)均为阴性,该标记物出现在尿路上皮癌中[28,29]。通过传代1年(约15代)的方法成功地建立了类器官的生物库,为今后的研究奠定了基础。尿酸生成成功与肿瘤分期无关(补充表1)。在一些患者中(n = 3) 在手术过程中,怀疑恶性肿瘤,但进一步的组织病理学检查发现只有良性组织(pT0N0)。从这些患者中建立类器官和类肿瘤细胞的尝试都失败了,突出了类器官生长培养基对膀胱癌细胞的选择性。  除了尿类物质无创取样的优点外,还有一些挑战和局限性:(1)与组织样本无菌手术室相比,基于尿液的方法由于在非无菌环境中进行自愿尿液取样,导致培养感染的几率更高。22例患者中有10例类器官培养物被细菌或酵母菌感染。随后,通过向培养基中添加特定的抗生素和抗真菌药物以及将每位患者的样本数量翻一番,解决了这个问题。这些变化将提高培养效率。(2) 尿苷类和肿瘤样培养物均缺乏肿瘤微环境(TME)成分。已经建立了几种可以表征肿瘤微环境成分的方法,例如与基质或免疫成分共培养的方法。当使用快速基于类器官的外植体技术(如MOS[48]或外植体[49]),允许在数周内进行药物筛选时,这一点尤其重要。在这些技术中,TME将存在于基于组织的培养物中,但不存在于尿液来源的培养物中。因此,这两个系统应使用互补或补充与孤立的细胞系的组成部分,以评估TME的贡献,疾病和治疗。(3) 尿酸和肿瘤样物质的建立和生物库都是耗时的。这种针对患者的类器官研究的内在局限性正在使用诸如前面提到的基于类器官的快速外植体技术等方法加以解决。  类器官为膀胱癌患者治疗期间培养连续随访样本提供了独特的机会。这些见解随后将指导针对耐药途径或后天脆弱性的当前和新的联合疗法的发展。最终,膀胱癌类器官是一种非侵入性工具,用于追踪膀胱癌的肿瘤发病机制、纵向药物反应监测和治疗适应性。 Urine-derived bladder cancer organoids (urinoids) as a tool for cancer longitudinal response monitoring and therapy adaptation Background Bladder cancer is one of the most common cancer types worldwide. Generally, research relies on invasive sampling strategies. Methods Here, we generate bladder cancer organoids directly from urine (urinoids). In this project, we establish 12 urinoid lines from 22 patients with non-muscle and muscle-invasive bladder tumours, with an efficiency of 55%. Results The histopathological features of the urinoids accurately resemble those of the original bladder tumours. Genetically, there is a high concordance of single nucleotide polymorphisms (92.56%) and insertions & deletions (91.54%) between urinoids and original tumours from patient 4. Furthermore, these urinoids show sensitivity to bladder cancer drugs, similar to their tissue-derived organoid counterparts. Genetic analysis of longitudinally generated tumoroids and urinoids from one patient receiving systemic immunotherapy, identify alterations that may guide the choice for second-line therapy. Successful treatment adaptation was subsequently demonstrated in the urinoid setting. Conclusion Therefore, urinoids can advance precision medicine in bladder cancer as a non-invasive platform for tumour pathogenesis, longitudinal drug-response monitoring, and therapy adaptation. Bladder cancer ranks among the top five and ten most common cancers worldwide in men and women, respectively [1]. Over 573.000 patients were diagnosed with bladder cancer worldwide in 2022 (IARC). Urothelial carcinoma (UCC) is the predominant histopathological subtype of bladder cancer (BC) and on initial staging presents as non-muscle-invasive bladder cancer (NMIBC; 73% of total) or muscle-invasive bladder cancer (MIBC) [2,3,4]. In general, NMIBC has a high recurrence rate (up to 84%), but rarely metastasizes, whereas MIBC is an aggressive disease with a high risk for relapse and metastases (up to 50%) [3, 4]. Current neoadjuvant cisplatin-based combination chemotherapy regimens result in a complete pathological response in around 25% of the cases [5]. Thus, evaluation of tumour biology and assessment of chemosensitivity of the individual bladder tumour is needed to guide personalised bladder cancer treatment [6,7,8,9,10,11]. Large-scale genetic analyses of bladder cancer have identified drivers such as TP53, ARID1A, PIK3CA, FGFR3, STAG2 and ERBB2 and have yielded several molecular classification methods [12], with consensus markers such as TP63 (Transitional/Intermediate cells), KRT5 (Basal class), KRT20 (Luminal class) and UP3A (Urothelial differentiation) [11, 13,14,15,16]. However, it remains challenging to apply such information in clinical practice to guide treatment decision-making [17]. In addition, drug treatment itself may induce changes in tumour characteristics, such as genetic instability, and cause enrichment of specific molecular subclones, which may result in altered drug sensitivity and acquired drug resistance [17,18,19,20]. Platforms that allow drug-response monitoring longitudinally may therefore have great value in developing personalised and adaptive treatment strategies. Urine as a source for non-invasive generation of bladder cancer organoids Bladder cancer patients from the University Medical Center Utrecht were invited to participate in this prospective proof-of-concept project by means of informed consent by the treating urologist or the nurse practitioner. Tumour tissue biopsies and urine samples were obtained from consenting bladder cancer patients, who underwent transurethral bladder tumour resection (TUR-BT) or a radical cystectomy. The urine samples were collected using a transurethral catheter (Fig. 1). Urine-derived cells were subsequently cultured in a bladder cancer organoid medium to generate organoids (urinoids) [16]. In parallel, bladder cancer organoids were generated from resected tumour tissue (tumoroids), which were also fresh-frozen for histopathological and genetic analyses. In total, 22 bladder cancer patients were included in this study, resulting in a living biobank of 12 urinoid lines (establishment efficiency 55%; Fig. 1b, Table 1 and Supplemental Table 1). Urinoids were generated from patients with various disease stages including NMIBC (Ta (8.3%); CIS (16.7%); T1 (50%)) and MIBC (T2 (16.7%); T3 (8.3%)) (Fig. 1b, Table 1 and Supplemental Table 1). Urinoid cultures capture the histopathological features of the original tumour tissue All urinoids, paired tumoroids and the original non-cultured tumour tissues were analysed by hematoxylin–eosin (HE) staining and by immunohistochemistry (IHC) to detect expression of bladder cancer subtype-specific and proliferation markers (Fig. 2 and Supplemental Fig. 1a–c). HE staining revealed that the histopathological morphology of the original tumour tissues was recapitulated in both the respective tumoroid and urinoid cultures (Fig. 2 and Supplemental Fig. 1c). IHC analysis of Ki67 by the pathologist determined that the percentage of positive (proliferative) tumour cells was highly similar among the original tumour tissues and paired tumoroids and urinoids (Fig. 2). Furthermore, expression of p53, p63, CK5 and CK20 was highly concordant among the original tumour tissues and paired tumoroids and urinoids (Fig. 2 and Supplemental Table 2). All original tissues, tumoroids and urinoids were negative for the nuclear kidney marker PAX8 (Supplemental Fig. 2). All tumour tissues and organoid lines were negative for the urothelial differentiation marker uroplakin 3a (UP3A)(Supplemental Fig. 2), which occurs in urothelial cancers [28, 29]. Urinoids were passaged for up to one year (~15 passages) and successfully biobanked via cryopreservation as a resource for future studies. Successful urinoid generation was not related to tumour stage (Supplemental Table 1). In several patients (n = 3), during surgery, malignancy was suspected, but upon further histopathological investigation revealed only benign tissue (pT0N0). Attempts for urinoid and tumoroid establishment from these patients all failed, highlighting the selectivity of the organoid growth medium for bladder cancer cells. Besides the benefits of the non-invasive sampling of urinoids, there are certain challenges and limitations: (1) the urine-based approach results in a higher chance of culture infection due to voluntary urine sampling in a non-sterile setting when compared to the sterile operation room setting for tissue samples. In ten out of twenty-two patients the urinoid cultures were infected with bacteria or yeast. This problem has subsequently been addressed with the addition of specific antibiotics and antimycotics to the culture medium and doubling the number of samples per patient. These changes will increase the culturing efficiency. (2) both urinoids and tumoroids cultures lack tumour microenvironment (TME) components. Several ways have been established that allow the representation of the tumour microenvironment components, such as coculture methods with stromal or immune components. This is especially relevant when rapid-organoid-based explant techniques are used (such as MOS [48] or Explant [49]), allowing drug screening within weeks. In such techniques, the TME will be present in tissue-based cultures, but not from urine-derived cultures. Thus, both systems should be used complementary or supplemented with isolated cell line components to assess the TME contribution to disease and therapies. (3) both urinoid and tumoroid establishment and biobanking are time-consuming. This intrinsic limitation of patient-specific organoid research is being addressed using methods such as the previously mentioned rapid-organoid-based explant techniques. Urinoids provide a unique opportunity to culture sequential follow-up samples from bladder cancer patients during their treatment. These insights will subsequently steer the development of current and novel combination therapies targeting drug-resistance pathways, or acquired vulnerabilities. Ultimately, urinoids are a non-invasive tool for following tumour pathogenesis, longitudinal drug-response monitoring, and therapy adaptation in bladder cancer. |
