Research Focus |
|
肌酐1,4,5三磷酸受体在禁食和糖尿病条件下调控肝脏糖异生 2012-12-26 09:45:05 浏览次数:1634 | |
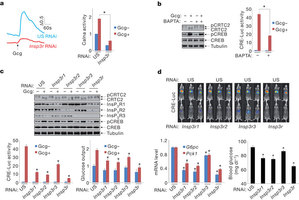
| 肌酐1,4,5三磷酸受体在禁食和糖尿病条件下调控肝脏糖异生 来源:仪方生物 www.yeslab.com 在禁食阶段,增高的循环胰高血糖素通过糖异生途径促进肝脏葡糖产生,环AMP通路通过CREB辅激活因子CRTC2去磷酸化激活糖异生相关的基因表达。胰高血糖素促进激酶A(PKA)介导的CRTC2激酶SIK2的抑制。一组丝氨酸/苏氨酸磷酸酶可能起着CRTC2去磷酸化的作用,但是荷尔蒙调控的机制并不清楚。作者在小鼠体内发现胰高血糖素通过迁移细胞间钙存储及活化钙/钙调蛋白依赖性的丝氨酸/苏氨酸磷酸酶钙调神经磷酸酶(也称为为PP3CA)来刺激CRTC2脱磷酸作用。胰高血糖素通过PKA介导的肌酐1,4,5三磷酸受体(InsP3Rs)促进细胞内钙离子浓度。活化之后,InsP3Rs通过启动钙调神经介导的CRTC2脱磷酸来增加糖异生基因的表达。在反馈途径中,增高的胰岛素信号利用AKT介导的InsP3Rs降低CRTC2活性。InsP3R活性在糖尿病中增高,从而诱发糖异生程序的上调。由于肝脏中的InsP3Rs下调及在胰岛素抗性中钙调神经磷酸酶促进循环的葡糖水平。这些结果表明在禁食和糖尿病中InsP3R调控肝脏葡萄糖产生中cAMP和钙通路的相互作用。 In the fasted state, increases in circulating glucagon promote hepatic glucose production through induction of the gluconeogenic program. Triggering of the cyclicAMP pathway increases gluconeogenic gene expression via the de-phosphorylation of the CREB co-activator CRTC2 (ref. 1). Glucagon promotes CRTC2 dephosphorylation in part through the protein kinase A (PKA)-mediated inhibition of the CRTC2 kinase SIK2. A number of Ser/Thr phosphatases seem to be capable of dephosphorylatingCRTC2 (refs 2, 3), but the mechanisms bywhich hormonal cues regulate these enzymes remain unclear.Here we show inmice that glucagon stimulatesCRTC2 dephosphorylation in hepatocytes by mobilizing intracellular calcium stores and activating the calcium/calmodulin-dependent Ser/Thr-phosphatase calcineurin (also known as PP3CA). Glucagon increased cytosolic calcium concentration through the PKA-mediated phosphorylation of inositol-1,4,5-trisphosphate receptors (InsP3Rs), which associate with CRTC2. After their activation, InsP3Rs enhanced gluconeogenic gene expression by promoting the calcineurinmediated dephosphorylation of CRTC2. During feeding, increases in insulin signalling reduced CRTC2 activity via the AKT-mediated inactivation of InsP3Rs. InsP3R activity was increased in diabetes, leading to upregulation of the gluconeogenic program. As hepatic downregulation of InsP3Rs and calcineurin improved circulating glucose levels in insulin resistance, these results demonstrate how interactions between cAMP and calcium pathways at the level of the InsP3R modulate hepatic glucose production under fasting conditions and in diabetes. |