Research Focus |
|
乳腺癌类器官活体生物库捕获疾病异质性 2024-04-01 11:14:37 浏览次数:509 | |
| 乳腺癌类器官活体生物库捕获疾病异质性 乳腺癌(BC)包括多种不同的亚型,这些亚型在遗传、病理和临床上都不同。在这里,我们描述了一个强大的协议,长期培养人乳腺上皮类器官。使用该方案,产生了100个以上的原发性和转移性BC类器官系,大致概括了疾病的多样性。BC类器官形态通常与原始肿瘤的组织病理学、激素受体状态和HER2状态相匹配。DNA拷贝数的变化和序列的变化在肿瘤类器官对内是一致的,即使在延长传代后也基本保持不变。BC类类器官进一步填充了所有主要的基于基因表达的分类组,并允许进行与体内异种移植和患者反应一致的体外药物筛选。这项研究描述了一个具有代表性的收集良好的特点BC类类器官可用于癌症研究和药物开发,以及一个战略,以评估体外药物反应的个性化方式。 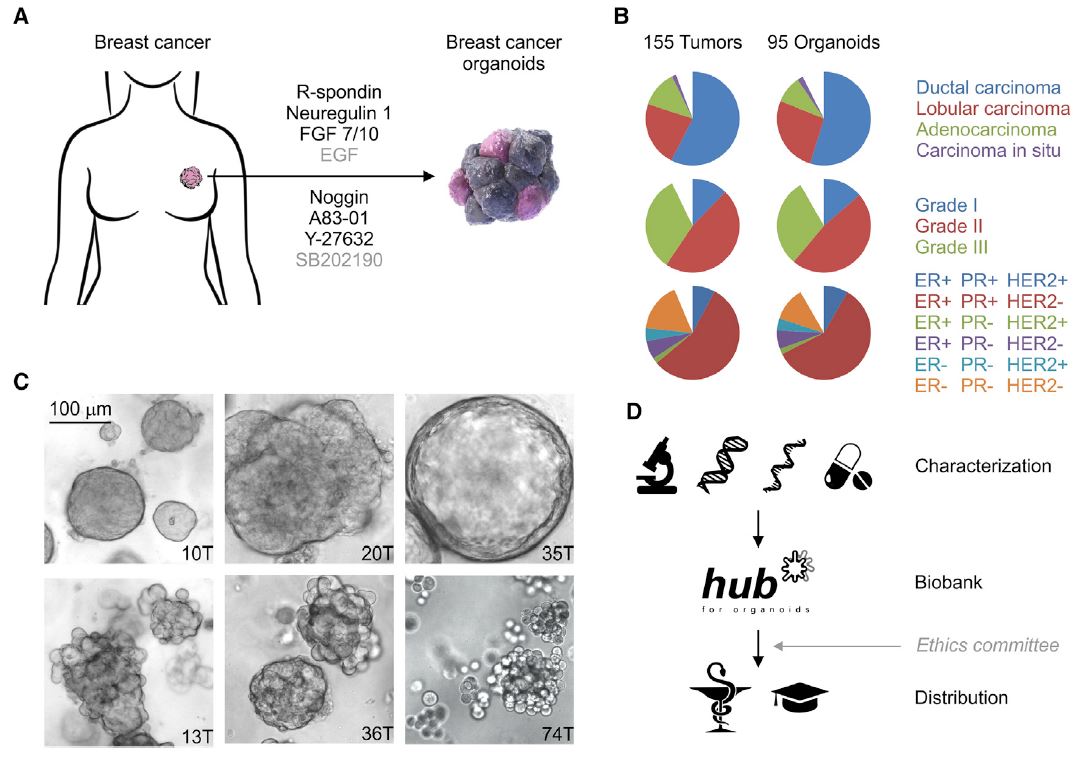 乳腺癌(BC)是全世界女性中最常见的癌症和癌症死亡原因(Stewart和Wild,2014)。BC包括20多个不同的亚型,这些亚型在遗传、形态学和临床上存在差异(Lakhani,2012)。下一代测序研究绘制了全面的BC分子图谱(Cancer Genome Atlas,2012;Ciriello et al.,2015),并在93个BC基因中确定了1600多个可能的驱动突变(Nik Zainal et al.,2016),最能说明BC异质性。尽管人们越来越了解BC的复杂性,但目前的治疗方法主要基于BC的临床和病理特征,辅以激素受体和人类表皮生长因子受体2(HER2)状态(Lakhani,2012)。因此,目前的标准系统疗法(激素、细胞毒性和HER2靶向疗法)不能很好地适应个体患者。作为回应,临床试验正在评估合理选择的靶向治疗(Zardavas等人,2013年),个体化治疗是最终目标。然而,单独识别独特的分子肿瘤特征只能指导治疗,如果没有功能验证,将无法预测临床结果。反之亦然,即使在没有分子知识的情况下,对BC药物进行体外功能测试也可以预测患者的预后。 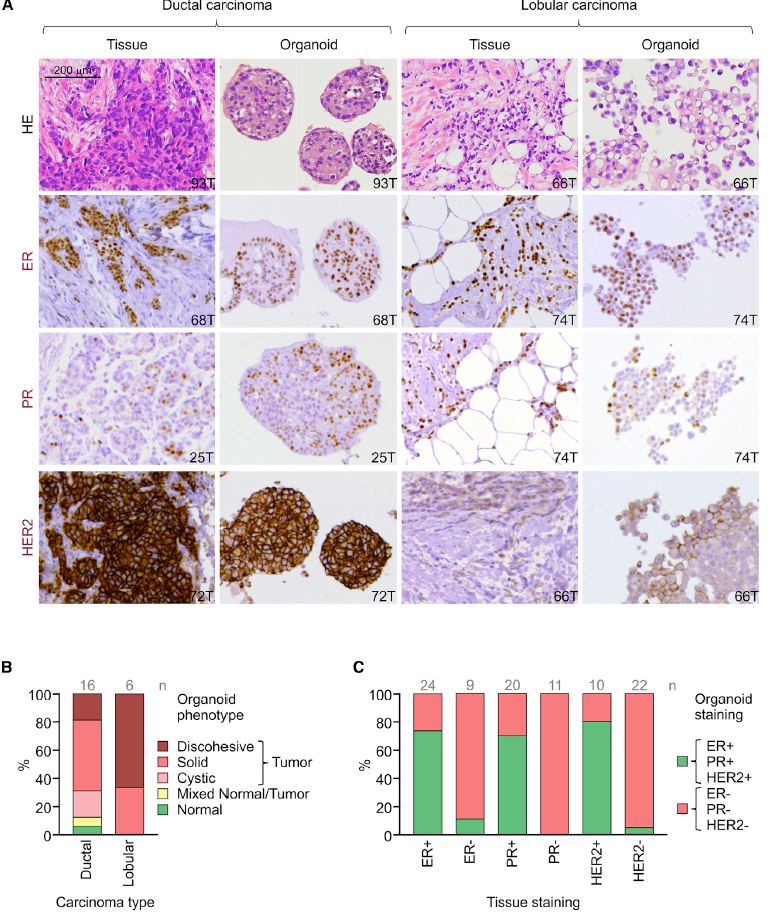 我们从知情同意下接受肿块切除术(表S1)的患者获得BC组织,移除正常组织,并通过机械破坏和酶消化的组合分离BC细胞(参见STAR方法)。将分离的细胞接种在贴壁BME滴液中,并用优化的BC类有机培养基覆盖(图1A;表S2)。与先前建立的人类类器官方案(Sato和Clevers,2015;Sato等人,2011)相比,一个关键的补充是有丝分裂原神经调节蛋白1。神经调节蛋白1是人类表皮生长因子受体(HER)酪氨酸激酶-3和-4的配体,与乳腺发育和肿瘤发生有关(Roskoski,2014;Troyer和Lee,2001;Wansbury等人,2008;Yang等人,1995)。将其添加到BC类类器官培养基中,可使BC类类器官高效产生,并可长期扩增20代以上。抑制Rho相关的含有蛋白激酶(ROCK)的卷曲线圈已被证明可使肿瘤上皮细胞在体外长期增殖(Liu等人,2012年),并且添加特异性ROCK抑制剂Y-27632确实改善了培养条件。在培养基优化过程中,我们进一步注意到(1)Wnt-3A的加入并没有显著改善培养条件,(2)EGF浓度高于5 ng 3 mlu 1时,增加了细胞增殖,但导致BC类类器官通过BME逐渐下沉并失去其三维结构,(3)SB202190浓度高于1 mM时,降低了类类器官建立的效率。 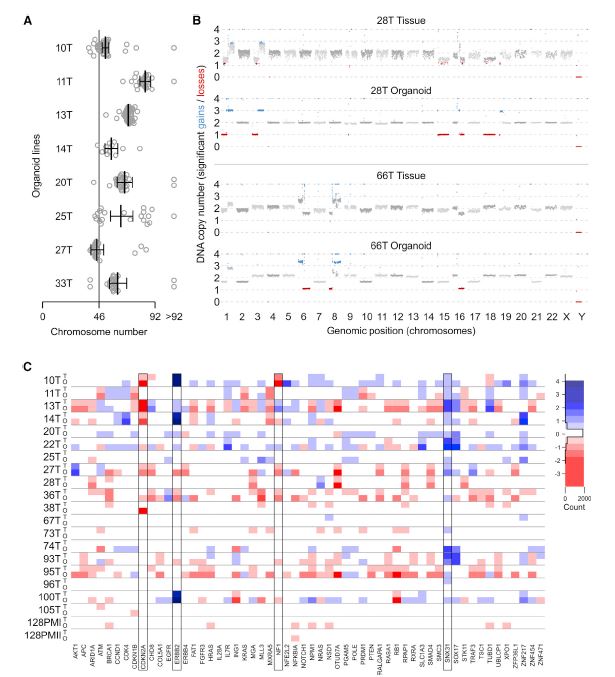 BC类类器官的第三个关键特征是能够进行功能性高通量药物筛选,如之前针对CRC类类器官所证明的(van de Wetering et al.,2015)。重要的是,体外获得的药物敏感性似乎具有生理学意义,如异种移植试验的概念证明和转移性BC患者对衍生BC类器官系他莫昔芬反应的概述所示。为了明确地翻译这些发现,类器官应从BC活检中产生,并在规定的临床试验中与相应患者并行治疗。理想情况下,BC类类器官可以帮助确定个别患者可能的治疗策略和耐药性,即使在缺乏分子基础的情况下。事实上,CRC类有机药物筛选最近已用于功能性识别靶向治疗的耐药机制(Verissimo等人,2016)。将组织学、遗传和/或基因表达数据与BC类有机药物反应相关将进一步推进我们对BC的分子和功能理解。 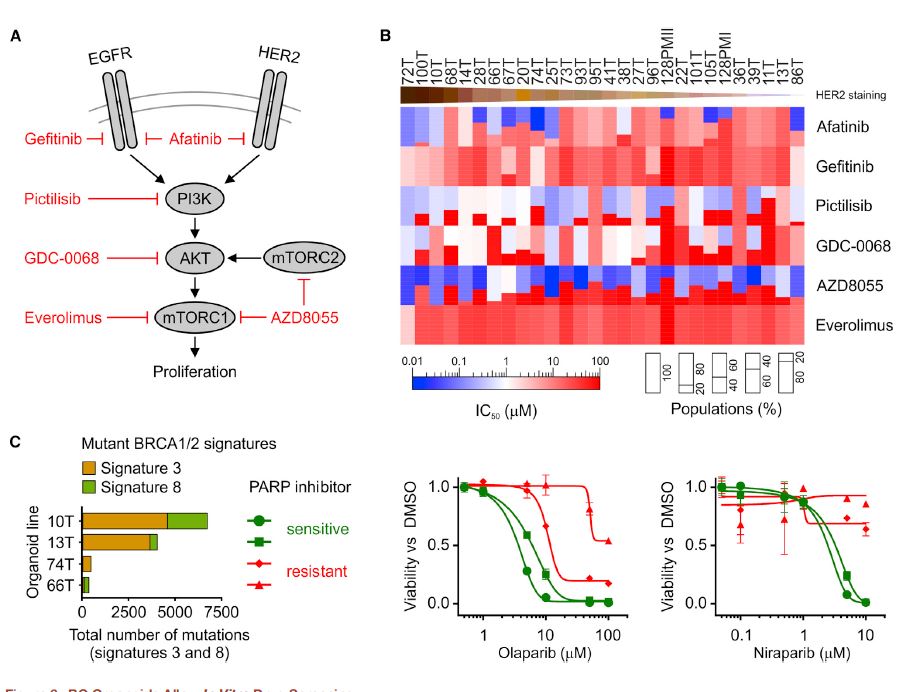 为了在伦理上负责任地与科学界共享已建立的BC类器官线和相关数据,同时保护患者保密性和撤销知情同意的权利,HUB和METC-UMCU实施了严格的协议,监督生物库和个案分配。我们已经从各种原发性和转移性肿瘤中无偏见地建立了100多个BC类器官细胞系的活体生物库,并将继续这样做,直到我们达到一个代表所有BC亚型的全面BC类器官集。总之,我们建议BC类类器官作为有价值的临床前BC模型,用于学术、临床和药学研究。 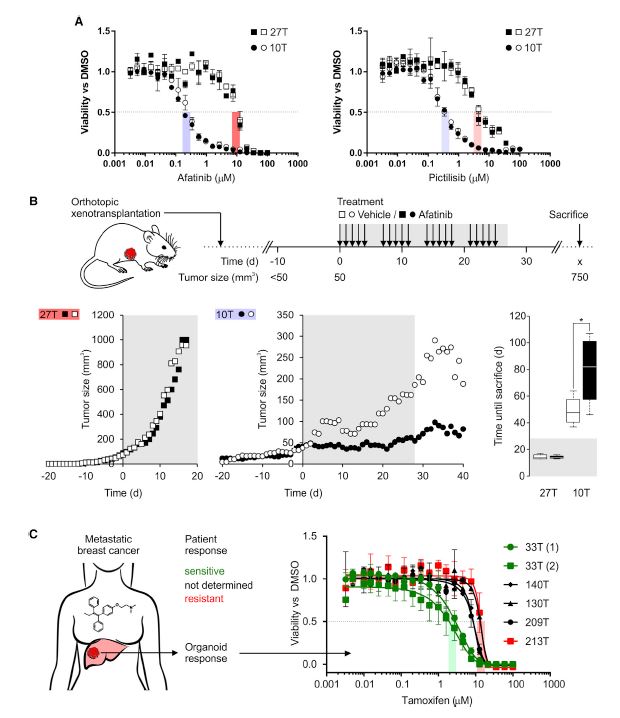 A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity Breast cancer (BC) comprises multiple distinct subtypes that differ genetically, pathologically, and clinically. Here, we describe a robust protocol for long-term culturing of human mammary epithelial organoids. Using this protocol, >100 primary and metastatic BC organoid lines were generated, broadly recapitulating the diversity of the disease. BC organoid morphologies typically matched the histopathology, hormone receptor status, and HER2 status of the original tumor. DNA copy number variations as well as sequence changes were consistent within tumor-organoid pairs and largely retained even after extended passaging. BC organoids furthermore populated all major gene-expression- based classification groups and allowed in vitro drug screens that were consistent with in vivo xeno-transplantations and patient response. This study describes a representative collection of well-characterized BC organoids available for cancer research and drug development, as well as a strategy to assess in vitro drug response in a personalized fashion. Breast cancer (BC) is the most frequently diagnosed cancer and cause of cancer death among women worldwide (Stewart and Wild, 2014). BC comprises more than 20 distinct subtypes that differ genetically, morphologically, and clinically (Lakhani, 2012). BC heterogeneity is best exemplified by next-generation sequencing studies that have drawn comprehensive molecular BC portraits (Cancer Genome Atlas, 2012; Ciriello et al., 2015) and identified more than 1,600 likely driver mutations in 93 BC genes (Nik-Zainal et al., 2016). Despite the increased understanding of BC complexity, therapeutic approaches are currently largely based on clinical and pathological BC features, supplemented by hormone receptor and human epidermal growth factor receptor 2 (HER2) status (Lakhani, 2012). Consequently, the present standard systemic therapies (hormone, cytotoxic, and HER2-targeted) are unsatisfactorily tailored to individual patients. In response, rationally chosen targeted therapies are being evaluated in clinical trials (Zardavas et al., 2013), with individualized therapy being the final goal. Identifying unique molecular tumor features alone, however, can only guide treatment and will fail to predict clinical results without functional validation. Vice versa, functionally testing BC remedies in vitro could predict patient outcome even without molecular knowledge. We obtained BC tissue from patients that underwent lumpectomy under informed consent (Table S1), removed normal tissue, and isolated BC cells through a combination of mechanical disruption and enzymatic digestion (see the STAR Methods). Isolated cells were plated in adherent BME drops and overlaid with optimized BC organoid culture medium (Figure 1A; Table S2). A key addition, compared to previously established human organoid protocols (Sato and Clevers, 2015; Sato et al., 2011), was the mitogen Neuregulin 1. Neuregulin 1 is a ligand of human EGF receptor (HER) tyrosine kinases -3 and -4 and has been implicated in mammary development as well as tumorigenesis (Roskoski, 2014; Troyer and Lee, 2001; Wansbury et al., 2008; Yang et al., 1995). Its addition to BC organoid medium allowed the efficient generation of BC organoids as well as their long term expansion for >20 passages. Inhibition of Rho-associated coiled-coil containing protein kinase (ROCK) has been shown to allow long-term proliferation of tumor epithelial cells in vitro (Liu et al., 2012) and addition of the specific ROCK inhibitor Y-27632 indeed improved culture conditions. During medium optimization we furthermore noted that (1) addition of Wnt-3A did not improve culture conditions notably, (2) EGF concentrations above 5 ng 3 ml_1 increased proliferation, but caused BC organoids to gradually sink through BME and lose their three dimensional organization, and (3) SB202190 concentrations above 1 mM decreased efficiency of organoid establishment. The third critical feature of BC organoids is the possibility to perform functional high-throughput drug screens, as demonstrated earlier for CRC organoids (van de Wetering et al., 2015). Importantly, drug sensitivities obtained in vitro appear to bear physiological significance as shown here in proof of concept xeno-transplantation assays and recapitulation of the tamoxifen response of metastatic BC patients by derived BC organoid lines. In order to unequivocally translate these findings, organoids should be generated from BC biopsies and treated in parallel to the respective patients in defined clinical trials. Ideally, BC organoids could aid in identifying possible treatment strategies and resistances for individual patients even in the absence of molecular underpinnings. Indeed, CRC organoid drug screens have recently been used to functionally identify resistance mechanisms of targeted therapies (Verissimo et al., 2016). Correlating histological, genetic, and/ or gene expression data to BC organoid drug responses will further advance our molecular and functional understanding of BC. In order to share established BC organoid lines and relevant data ethically responsibly with the scientific community, while protecting patient confidentiality and the right to withdraw informed consent, HUB and METC-UMCU implemented a rigorous protocol overseeing biobanking and case-by-case distribution. We already built a living biobank of more than 100 BC organoid lines from a wide variety of primary and metastatic tumors without bias and will continue to do so until we reached a comprehensive BC organoid set representative of all BC subtypes. In conclusion, we propose BC organoids as valuable pre-clinical BC model for academic, clinical, and pharmaceutical research. |