Research Focus |
|
直肠癌类器官平台研究个体化放疗反应 2024-04-01 11:14:37 浏览次数:506 | |
| 直肠癌类器官平台研究个体化放疗反应 直肠癌(RC)是一种具有挑战性的疾病,需要化疗、放疗和手术来优化个体患者的预后。没有精确的RC模型来回答与患者相关的基础研究问题。我们建立了一个由65例原发性、转移性或复发性疾病患者来源的RC类器官培养物(肿瘤样物)的生物储存库。RC类肿瘤保留了其来源肿瘤的分子特征,其对临床相关化疗和放疗的体外反应与个别患者肿瘤的临床反应相关。人结直肠癌移植到小鼠直肠黏膜后,可发生侵袭性结直肠癌,并向肺、肝转移。重要的是,移植瘤表现出异质性化疗敏感性的临床观察。因此,RC临床分离株的生物学和药物敏感性可以通过基于类类器官的体外平台和动物体内腔内繁殖进行有效的检测。 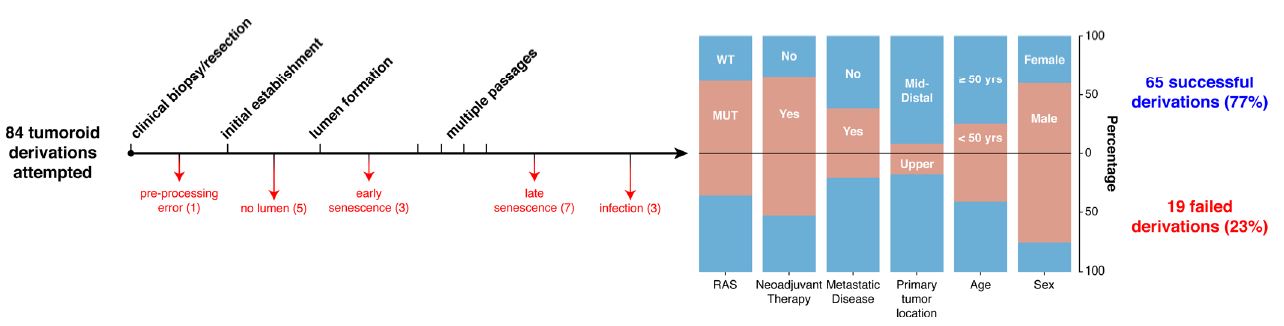 在美国,结肠癌和直肠癌(RCs)每年造成超过50000人死亡1。RC尤其具有挑战性,因为与结肠癌2相比,诊断后的治疗更为复杂,因为肿瘤位于骨盆,且靠近重要的泌尿生殖器官。侵袭直肠周围组织或淋巴结的RCs采用三模式治疗,包括新辅助放化疗(CRT)、手术切除和基于5-氟尿嘧啶(5-FU)的化疗2。一些RC患者单独使用CRT完全有效,可以完全避免手术3–6,但其他患者反应差,需要根治性手术7。前瞻性识别在单独新辅助治疗后能达到完全缓解7,8的患者,将使个体化治疗方案更具针对性9,10,从而将过度治疗的潜在危害降至最低。临床反应的异质性和与根治性手术相关的发病率强调需要更复杂的模型来预测对标准治疗的反应。 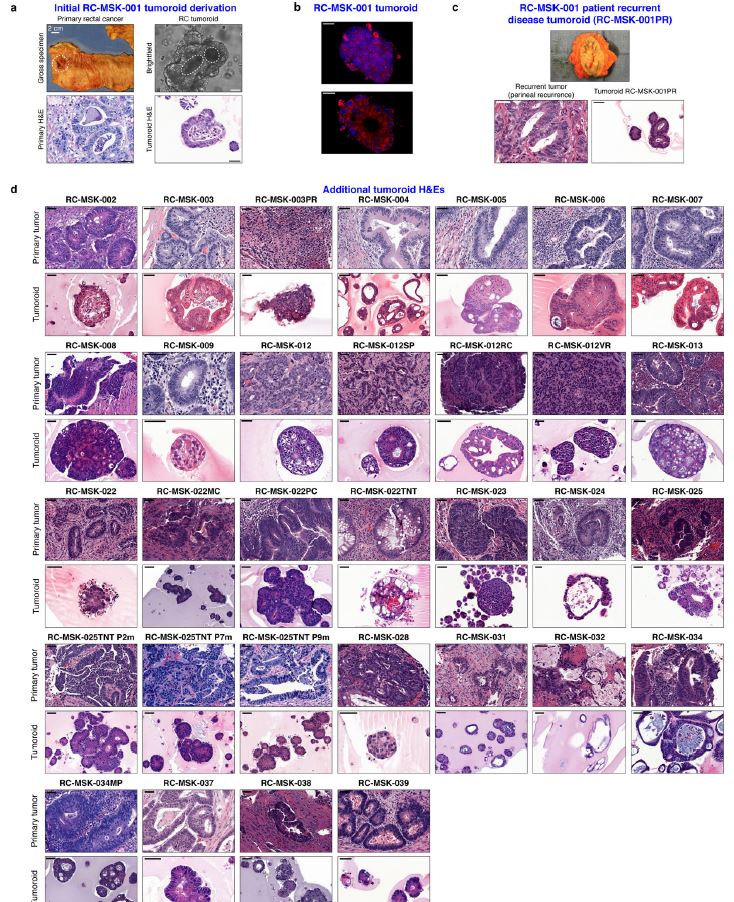 从RCs11–15中获得的细胞系很少,并且它们是否来自真正的解剖直肠和/或来自接受多模式治疗的患者尚不可能得到证实。尽管在新辅助治疗中使用三模式疗法治疗RC与结肠癌不同,但RC治疗的临床前发展一直依赖结肠癌细胞系16,这突出了开发RC特异性模型的必要性。此外,获得类类器官“生物库”的努力主要集中在结肠癌标本17上,RC组织或类类器官的专用生物库仍然是该领域未满足的需求。  鉴于异种移植物模型的缺乏18和腔内RC模型的完全缺乏,RC研究中还需要使用患者来源的RC类器官建立解剖精确的体内模型。直肠通过髂血管有独特的静脉引流,主要引起肺转移19(69%),肝转移较少(20%),这在结肠癌中更常见。鉴于我们的研究组20和其他研究组21最近成功地将小鼠结肠癌细胞移植到结肠腔内,我们着手从切除或活检的RCs中提取RC类器官(以下简称“肿瘤样物”),并使用它们建立体外和体内RC模型。我们研究这类肿瘤模拟人类RCs的分子和组织学特征,以及肿瘤发生、侵袭和转移的能力。我们还研究了我们的体外和体内平台是否可用于在可能告知临床治疗决定的时间范围内与个体患者的治疗反应相关。 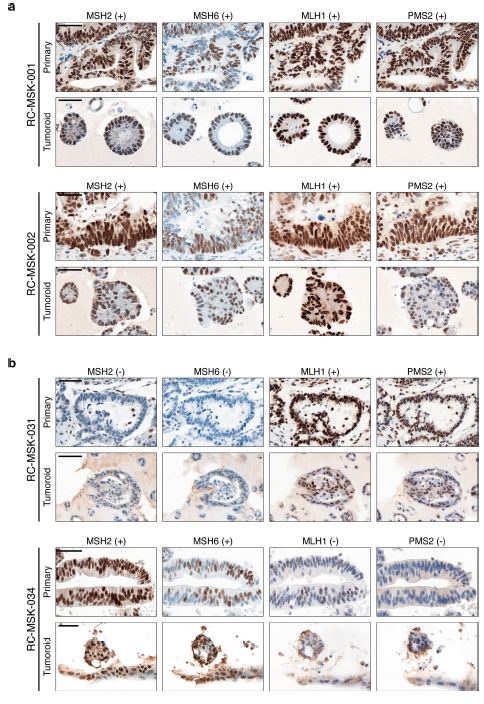 为了生成能够反映单个患者肿瘤生物学特性的RC模型,我们采用现有的3D离体肿瘤培养策略22,23,从治疗前后患者样本中生成RC肿瘤样物。扩展数据图1显示了我们试图从中衍生肿瘤样物的所有患者的基本特征。通过对58例患者进行84次肿瘤衍生试验,我们从41例患者中建立了65个RC肿瘤,总成功率为77%(65/84)。除了这些RC类肿瘤外,我们还从正常邻近组织生成51个正常直肠类器官(见补充表1)。在19次失败的类肿瘤尝试中,7次在衰老前维持6周。与以往在结肠癌中的研究类似,RC类肿瘤可以在缺少关键生长因子17(例如R-spondin、Wnt-3a和Noggin)的情况下培养,而来源于正常直肠粘膜的类器官仍然依赖于生长因子23。 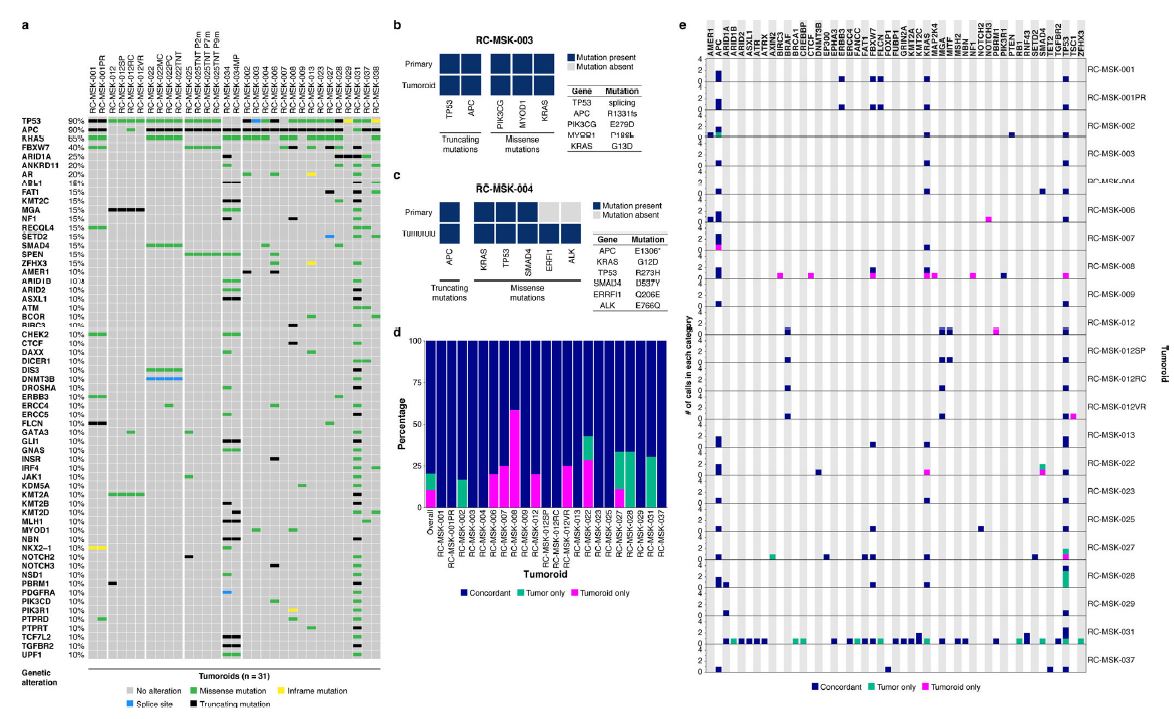 由于内镜活检是在门诊治疗RC患者的常规方法,我们询问是否可以从临床获得的微量材料中获得RC类肿瘤。值得注意的是,49个RC类肿瘤是使用临床护理中常规使用的活检钳获得的组织建立的(图1a)。这些数据表明,在治疗前或治疗后,可以使用作为标准护理的一部分获得的连续活检来生成肿瘤样模型,以评估患者特异性的反应和耐药性介质。 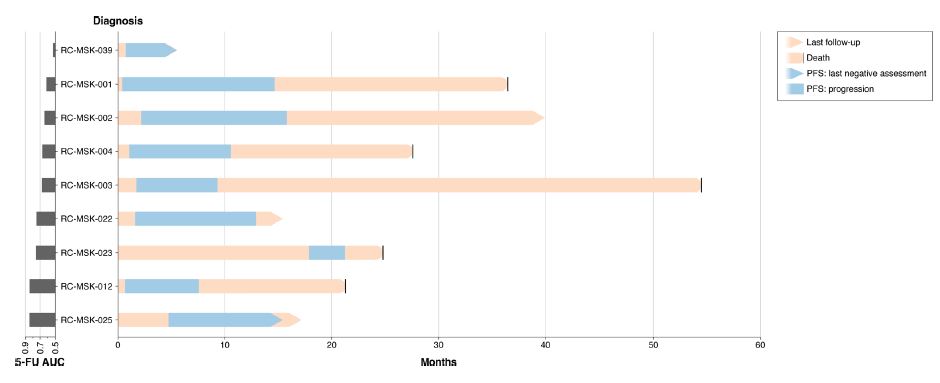 这项工作的另一个新颖之处在于建立了腔内RC模型,该模型可以概括对治疗的临床反应。该模型建立在我们最近的小鼠移植模型20的基础上,但也是第一次证明经治疗和未经治疗的人类RC肿瘤可以在小鼠直肠内移植,并再现原发疾病形成、随后的侵袭和最终转移的过程。值得注意的是,我们观察到转移的发展,这可能标志着来源于肿瘤的人类RCs的趋向性。虽然这些发现很有趣,但需要在更大的队列中进行前瞻性验证。总之,这些数据表明,我们已经建立了一个工作的RC平台,以更好地阐明RC的发病机制,并解决有关化疗和放疗耐药性的问题。 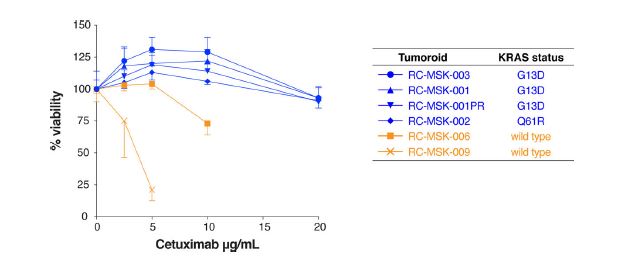 总之,我们的工作解决了一个未满足的需要,通过建立一个RC特异性的体外和体内生物反应器,显著提高了研究RC的能力。我们的模型在分子上类似于RC,并建立了一个研究该疾病的相关框架。该方法展示了临床前最终药物筛选37的选择,并反映了患者的临床结果。我们的研究表明,在推导的几周内确定基本的反应参数,可以作为治疗反应建模的工具,从而我们可以研究与个别RC患者相关的基础研究问题。 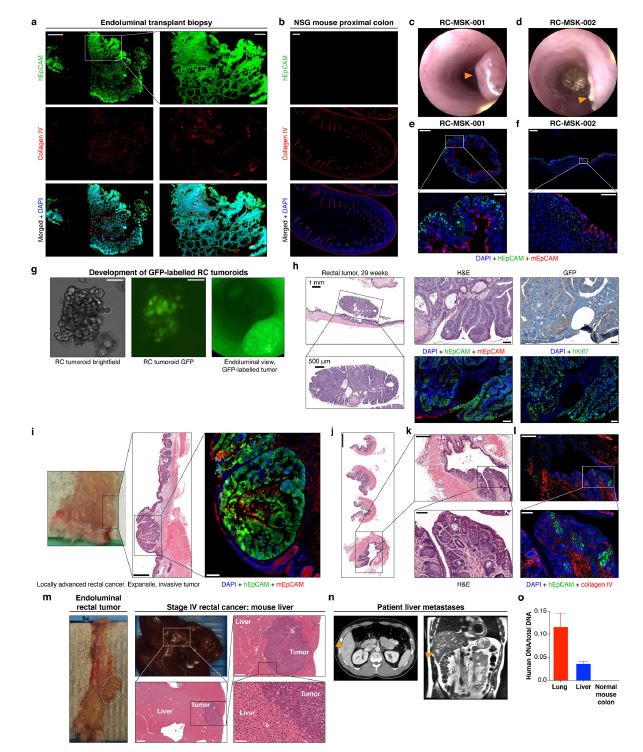 A rectal cancer organoid platform to study individual responses to chemoradiation Rectal cancer (RC) is a challenging disease to treat that requires chemotherapy, radiation, and surgery to optimize outcomes for individual patients. No accurate model of RC exists to answer fundamental research questions relevant to patients. We established a biorepository of 65 patient-derived RC organoid cultures (tumoroids) from patients with primary, metastatic, or recurrent disease. RC tumoroids retained molecular features of the tumors from which they were derived, and their ex vivo responses to clinically relevant chemotherapy and radiation treatment correlated with the clinical responses noted in individual patients’ tumors. Upon engraftment into murine rectal mucosa, human RC tumoroids gave rise to invasive RC followed by metastasis to lung and liver. Importantly, engrafted tumors displayed the heterogenous sensitivity to chemotherapy observed clinically. Thus, the biology and drug sensitivity of RC clinical isolates can be efficiently interrogated using an organoid-based, ex vivo platform coupled with in vivo endoluminal propagation in animals. Colon and rectal cancers (RCs) are responsible for > 50,000 deaths per year in the United States1. RC is particularly challenging, as treatment after diagnosis is more complex compared to colon cancer2 due to tumor location in the pelvis and close proximity to critical genitourinary organs. RCs invading the perirectal tissues or lymph nodes are treated with tri-modal therapy, which consists of neoadjuvant chemoradiation (CRT), surgical resection, and 5-fluorouracil (5-FU)-based chemotherapy2. Some RC patients respond completely to CRT alone and can avoid surgery entirely3–6, but others respond poorly and require radical surgery7. Prospective identification of patients who would achieve a complete response7,8 after neoadjuvant therapy alone would enable more tailored individual treatment regimens9,10 and thereby minimize potential harm from overtreatment. The heterogeneity in clinical response and the morbidity associated with radical surgery highlight the need for more sophisticated modeling to predict response to standard therapies. Few cell lines have been derived from RCs11–15 and whether they were derived from the true anatomic rectum and/or from patients undergoing multimodal therapy is impossible to confirm. Despite the fact that RC is treated differently from colon cancer by using tri-modal therapy in a neoadjuvant context, the preclinical development of treatments for RC has historically relied on colon cancer cell lines16, highlighting the need to develop RC-specific models. Furthermore, efforts to derive organoid “biobanks” have focused primarily on colon cancer specimens17, with a dedicated biorepository of RC tissue or organoids remaining an unmet need in the field. Given the paucity of xenografts models18 and the complete lack of endoluminal RC models, there is an additional need in RC research for an anatomically accurate in vivo model using patient-derived RC organoids. The rectum has unique venous drainage via the iliac vessels that gives rise predominantly to lung metastases19 (69%), and less frequently liver metastases (20%), which are more commonly seen in colon cancers. Given recent success transplanting mouse colon cancer cells into the colon lumen by our group20 and others21, we set out to derive RC organoids (hereafter “tumoroids”) from resected or biopsied RCs and use them to establish ex vivo and in vivo RC models. We investigate the ability of such tumoroids to model the molecular and histologic features of human RCs, as well as tumor initiation, invasion, and metastasis. We also investigate whether our ex vivo and in vivo platforms could be used to correlate with treatment response in individual patients within a time frame that could potentially inform clinical treatment decisions. With the goal of generating RC models that would reflect the biology of an individual patient’s tumor, we adapted existing strategies for 3D ex vivo tumor culture22,23 to generate RC tumoroids from pre- and post-treatment patient samples. Basic characteristics of all patients from whom we attempted tumoroid derivation are shown in Extended Data Fig. 1. After 84 tumoroid derivation attempts from 58 individual patients, we established 65 RC tumoroids from 41 patients with an overall success rate of 77% (65/84). In addition to these RC tumoroids, we also generated 51 normal rectal organoids from normal adjacent tissue (see Supplementary Table 1). Of the 19 failed tumoroid attempts, seven were maintained for 6 weeks before senescing. Similar to prior work in colon cancer, RC tumoroids could be cultured in the absence of key growth factors17 (e.g., R-spondin, Wnt-3a, and Noggin), whereas organoids derived from normal rectal mucosa remained growth factor dependent23. Since endoscopic biopsies are routinely performed in the outpatient setting in the treatment of patients with RC, we asked whether we could derive RC tumoroids from minute amounts of material obtained in the clinic. Of note, 49 of the RC tumoroids were established using tissue obtained with biopsy forceps routinely used in clinical care (Fig. 1a). These data suggest it is possible to use serial biopsies obtained as part of standard care in the pre-treatment or post-therapy settings to generate tumoroid models to assess patient-specific mediators of response and resistance. Additional novelty of this work lies in the establishment of the endoluminal RC model that recapitulates clinical response to therapy. This model builds on our recent murine model of transplantation20, but is also the first demonstration that treated and untreated human RC tumors can engraft within the mouse rectum and recapitulate the process of primary disease formation, subsequent invasion, and eventual metastasis. Notably, we observed the development of metastases that may signal tropism of the human RCs from which the tumoroids were derived. While these findings are intriguing, they require prospective validation in a larger cohort. Overall, these data indicate that we have established a working RC platform to better elucidate RC pathogenesis and address questions regarding resistance to chemotherapy and radiation. In summary, our work addresses an unmet need by establishing a RC-specific ex vivo and in vivo biorepository that markedly increases the ability to study RC. Our model molecularly resembles RC and establishes a relevant framework in which to study the disease. This methodology demonstrates options for eventual drug screening37 in a pre-clinical setting and reflects the clinical outcomes of the patients from which they were derived. Our study demonstrates determination of basic response parameters within weeks of derivation and can serve as a tool for therapeutic response modeling whereby we can study fundamental research questions relevant to individual RC patients. |
