Research Focus |
|
利用类器官对胃肠道进行逆向工程的挑战与展望 2024-04-01 11:14:37 浏览次数:517 | |
| 利用类器官对胃肠道进行逆向工程的挑战与展望 来源:仪方生物 www.yeslab.com 十多年来,类器官模拟消化系统的发育、生理和疾病一直是人们广泛关注和深入研究的课题。由于每个组织替代物都需要对原始方案进行量身定制的修改以生成肠类器官,因此建立能够再现胃肠道(GIT)所有不同区域的类器官模型具有挑战性。本文综述了GIT类类器官模型面临的挑战和研究进展。此外,我们设想下一代GIT类类器官作为完整的类类器官模型,能够在单个体外模型中再现消化管多个区域的结构和功能特征。我们讨论了这些新的趋势,并对GIT体外模型的未来进行了展望。 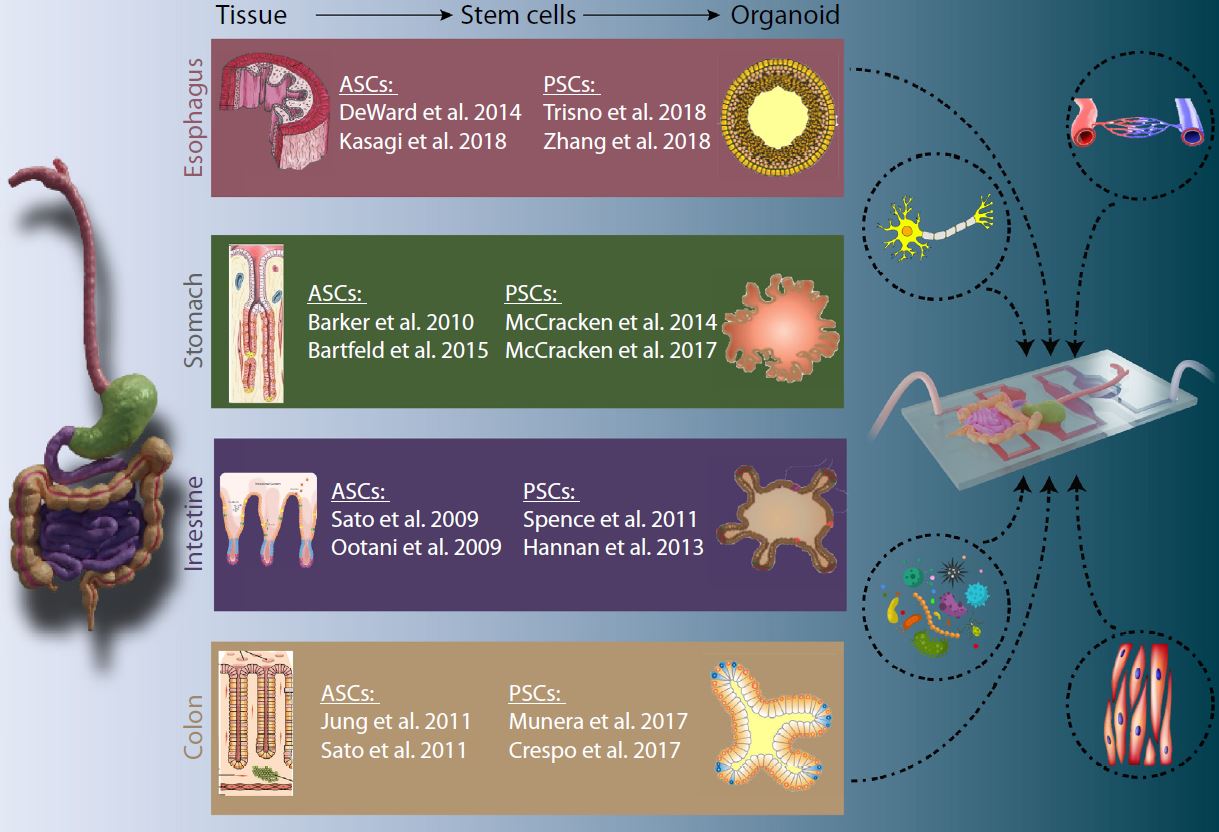 类器官模型的出现和迅速发展为干细胞研究领域做出了重要贡献。类器官来源于两种主要的干细胞类型:多能干细胞(PSCs;参见术语表)或器官特异性成体干细胞(ASCs)。对于PSC衍生模型,胚胎干细胞(ESCs)和诱导多能干细胞(iPSCs)均可使用。由此产生的类类器官包含上皮细胞和间充质细胞类型或仅包含上皮细胞[1–4]。ASC衍生模型主要用于疾病建模、个体化治疗以及研究组织再生和稳态,而PSC衍生模型主要用于研究发育和发育障碍。这些自组织三维模型是填补体外单层培养和体内研究之间差距的有希望的工具,因为它们比传统的二维细胞培养更准确地再现组织功能和结构。尽管它们具有结构和功能复杂性增加的特点,但它们符合大多数标准实验技术[5–8]。在这篇综述中,我们重点讨论了GIT的类器官模型,并讨论了通过使用最新开发的技术和生物工程方法(图1,关键图)进一步将这些模型近似于复杂的多区域消化道的潜在未来方向。 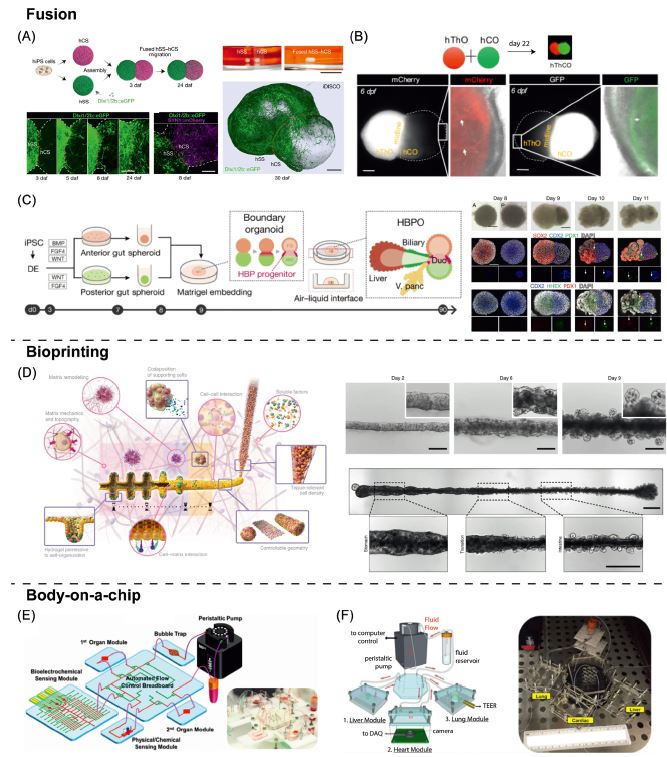 该融合方法已初步应用于脑类器官领域。2017年,建立了前三个融合前脑类器官模型,显示了它们在发育过程中皮质中间神经元迁移研究中的潜力[75–77]。在所有三种情况下,两个区域特异性类器官组装成一个多区域神经3D微生理系统,维持类似于体内的空间结构和功能(图2A)。同样,丘脑类器官与皮质类器官融合,建立丘脑-皮质相互轴突投射,类似于其体内对应物[78](图2B)。这些研究为产生更先进的类器官系统奠定了基础,这些系统不仅能够模拟大脑发育过程中发生的复杂相互作用,而且能够模拟某些疾病。在第一个与消化系统相关的综合模型中,融合了前肠和中肠球体(图2C)。由此产生的球体嵌入并在Matrigel穹隆中成熟,Matrigel穹隆是从两个球体之间的边界分支出的肝胆胰类器官[79]。这些自身模式的多器官结构再现了肝胆胰器官发生前前肠和中肠边界内的体内相互作用。然而,我们可以预期胃/食管和肠的特征会出现在这个系统中,因为这些组织分别来自前肠和中肠。这表明该系统缺乏时空控制和引导机制,在未来的应用中需要更加严格的调控。然而,类似的方法也可以翻译到GIT的其他部分。例如,在后肠阶段,可使用相同的生长因子但不同暴露时间获得的近端和远端球体的融合可产生具有十二指肠、回肠甚至空肠特征的融合类器官结构,从而产生更完整和多区域的肠类器官模型。胃窦和胃底类器官的整合可使胃类器官更紧密地再现活体胃。此装配可在后肠前肠球体阶段进行,然后进行ECM封装。合成基质的空间分布模式有利于调控球体的融合,促进特定组织的发育,为适应局部微环境和支持不同类器官类型的生长提供了可能。考虑到每个组织替代物成熟和维持所需的不同生长因子,需要微流体和微流体平台来控制培养基供应并提供空间上不同的培养条件。前肠、中肠和后肠球体的空间控制的三重融合可以产生创新的类器官模型,因为消化系统的所有区域都来自原始肠道亚区。然而,为了实现时空控制的、有组织的融合,需要进一步调整使用流体动力聚焦、微流控捕获和释放、生物打印和机器人拾取和放置技术的更复杂技术,以适应芯片上类类器官的受控融合任务。在最近的一项研究中,研究人员使用3D生物打印技术生成具有体内结构和功能特征的宏观肠道结构[80]。胃和结肠上皮干细胞的连续打印导致了大管的形成,它不仅模仿器官特异性,而且模仿胃和肠之间的连接(图2D)。尽管这些基于类器官融合的更复杂类器官模型的建立方法已显示出有希望的结果,但此类组装超结构的形成机制仍需进一步研究。一个重要的考虑点是在这种集成系统中保持不同的组织边界,以避免上皮细胞或间充质细胞跨越这些边界迁移,从而“污染”相邻区域,从而使模型在生理上不那么相关和适用。这方面的技术应急计划可包括使用微流控装置,其中代表不同区域的类类器官在隔室中保持分离,类似于片上人体概念(图3A)。  Challenges to, and prospects for, reverse engineering the gastrointestinal tract using organoids For over a decade, organoids mimicking the development, physiology, and disease of the digestive system have been a topic of broad interest and intense study. Establishing organoid models that recapitulate all distinct regions of the gastrointestinal tract (GIT) has proven challenging since each tissue surrogate requires tailor-made modifications of the original protocol to generate intestinal organoids. In this review, we discuss the challenges and current advances of the GIT organoid models. Moreover, we envision the next-generation GIT organoids as integrated organoid models, able to recapitulate structural and functional characteristics of multiple regions of the digestive tube in a single in vitro model. We discuss these new trends and provide an outlook for the future of GIT in vitro models. The emergence of, and rapid progress in, organoid models have contributed significantly to the field of stem cell research. Organoids can be derived from two main stem cell types: pluripotent stem cells (PSCs; see Glossary) or organ-specific adult stem cells (ASCs). For PSC-derived models, both embryonic (ESCs) and induced pluripotent (iPSCs) stem cells can be used. The resulting organoids contain either both epithelial and mesenchymal cell types or only epithelial cells [1–4]. ASC-derived models are mostly used for disease modeling, personalized medicine, and to study tissue regeneration and homeostasis, whereas PSC-derived models are primarily used for studying development and developmental disorders. These self-organizing, 3D models are promising tools to bridge the gap between in vitro monolayer cultures and in vivo studies, because they recapitulate tissue function and structure more accurately than do conventional 2D cell cultures. Although they are characterized by an increased structural and functional complexity, they are amenable to most standard experimental techniques [5–8]. In this review, we focus on organoid models of the GIT and discuss the potential future directions to further approximate these models to the intricate complexity of a multiregional digestive tract by using recently developed technologies and bioengineering approaches (Figure 1, Key figure). The fusion method has primarily been applied in the field of brain organoids. In 2017, the first three models of fused forebrain organoids were established and showed their potential in the study of cortical interneuron migration during development [75–77]. In all three cases, the two region specific organoids assembled into a multiregion neural 3D microphysiological system that maintained a spatial architecture and function similar to in vivo (Figure 2A). Likewise, thalamic organoids were fused with cortical organoids establishing reciprocal thalamocortical axon projections similar to their in vivo counterparts [78] (Figure 2B). These studies laid the foundation for the generation of more advanced organoid systems, which are able to model not only complex interactions occurring in the brain during development, but also certain disorders. In the first integrated model related to the digestive system, a foregut and a midgut spheroid were fused (Figure 2C). The resulting spheroid was embedded and matured in a Matrigel dome, a hepatobiliary- pancreatic organoid branched from the boundaries between the two spheroids [79]. These self-patterned multiorgan structures recapitulate the in vivo interactions within the boundaries of foregut and midgut that precede hepato-biliary-pancreatic organogenesis. However, one would expect gastric/esophageal and intestinal characteristics to be present in this system since these tissues derive from the foregut and midgut, respectively. This indicates lack of spatiotemporal control and guiding mechanisms in the system, which should be more tightly regulated in future applications. Nevertheless, similar approaches could also be translated to other parts of the GIT. For instance, at the hindgut stage, a fusion of proximal and distal spheroids, which can be derived using the same growth factors but different durations of exposure, could produce fused organoid constructs featuring characteristics of the duodenum, ileum, and possibly even the jejunum, thus resulting in a more complete and multiregional intestinal organoid model. The integration of antral and fundic organoids could result in a gastric organoid recapitulating the in vivo stomach more closely. This assembly could be performed at the posterior foregut spheroid stage followed by ECM encapsulation. A spatial pattern of synthetic matrices, which offer the possibility to tailor the local microenvironment and support the growth of different organoid types, could benefit the control of the spheroid fusion and promote the development of specific tissues. Considering the different growth factors needed for the maturation and maintenance of each tissue surrogate, micro- and milli-fluidic platforms would be necessary to control the media supply and provide spatially distinct culture conditions. A spatially controlled, triple fusion of foregut, midgut, and hindgut spheroids could yield innovative organoid models, given that all regions of the digestive system derive from primitive gut subdomains. However, to accomplish a spatiotemporally controlled, organized fusion, more sophisticated techniques using, for example, hydrodynamic flow focusing, microfluidic trapping and releasing, bioprinting, and robotic pick-and-place techniques would need to be further adapted to the task of a controlled fusion of organoids on chips. In a recent study, researchers used 3D bioprinting to generate macro-scale intestinal constructs featuring structural and functional in vivo-like characteristics [80]. The sequential printing of stomach and colon epithelial stem cells led to the formation of large tubes, which mimicked not only organ-specific properties, but also the junction between stomach and intestine (Figure 2D). Although these methods to create more complex organoid models based on organoid fusion have demonstrated promising results, the mechanisms underlying the formation of such assembled superstructures require further investigation. An important point of consideration is the maintenance of distinct tissue borders in such integrated systems to avoid epithelial or mesenchymal cell migration across these borders, which would ‘contaminate’ the adjacent regions, thus making the model less physiologically relevant and applicable. A technical contingency plan for this could include the use of microfluidic devices in which the organoids representing different regions are kept separated in compartments, similar to body-on-a-chip concepts (Figure 3A). Organoids are one of the most exciting and rapidly advancing 3D in vitro models that have emerged over the past decade. The growing interest in more complex models placed organoids in a unique position as a tool for a range of applications. All the important regions of the GIT can be recapitulated separately by organoids representing both fetal and adult stages. Using organoids as building blocks to create more advanced integrated multiregion models appears to be the logical next step (see Outstanding questions). Methods such as organoid fusion, body-on-a-chip, and bioprinting have already shown the potential to establish such organoid-based superstructures. Adding to that and given the common developmental origins of these tissues, along with the similarities between the different organoid protocols, we foresee a significant opportunity to reverse engineer a complete GIT in vitro using organoids. Such a system would benefit both fundamental and applied research and would serve a variety of applications. |