Research Focus |
|
卵巢癌类器官的实验和临床前研究进展 2024-04-08 20:12:40 浏览次数:572 | |
| 卵巢癌类器官的实验和临床前研究进展 来源:仪方生物 www.yeslab.com 卵巢癌(OC)是妇科最常见的恶性肿瘤。病理生物学的了解甚少,主要是由于缺乏合适的研究模型。类器官是指组织在体外自我发展的三维重建,为人类疾病建模提供了强有力的工具。在此,我们从患者来源的OC,特别是最常见的高级别浆液性OC(HGSOC)中建立了类器官培养。通过检测多种培养基成分,确定神经调节蛋白-1(NRG1)是最大化OC类器官发育和生长的关键因素,尽管总体衍生效率仍然中等(HGSOC患者36%,所有患者44%)。所建立的类器官细胞系显示了患者肿瘤依赖性的形态学和疾病特征,并再现了亲本肿瘤的标记物表达和突变情况。此外,类有机物对临床HGSOC化疗药物具有肿瘤特异性敏感性。患者源性有机物为研究肿瘤的病理生物学(如NRG1/ERBB通路的重要性)提供了强有力的工具,也为(个性化)药物筛选和发现提供了先进的临床前工具。  卵巢癌(OC)是最致命的妇科肿瘤。在80%以上的患者中,该疾病直到晚期和转移时才被发现(Narod,2016)。初次去瘤手术和辅助化疗后,70%–80%的患者出现肿瘤复发,化疗耐药性增加(Pignata等人,2017年)。大多数OC病例表现为上皮表型(上皮OC[EOC]),75%的患者被诊断为FIGO III或IV期高级别浆液性OC(HGSOC)(即显示广泛转移性扩散)(Jelovac和Armstrong,2011)。HGSOC导致高达80%的OC患者死亡,因此是妇科肿瘤最突出的临床挑战。EOC的病因和起源部位,无论是卵巢表面上皮(OSE)还是输卵管上皮(FTE)(或两者兼而有之),仍处于激烈争论中(Kim等人,2018年)。 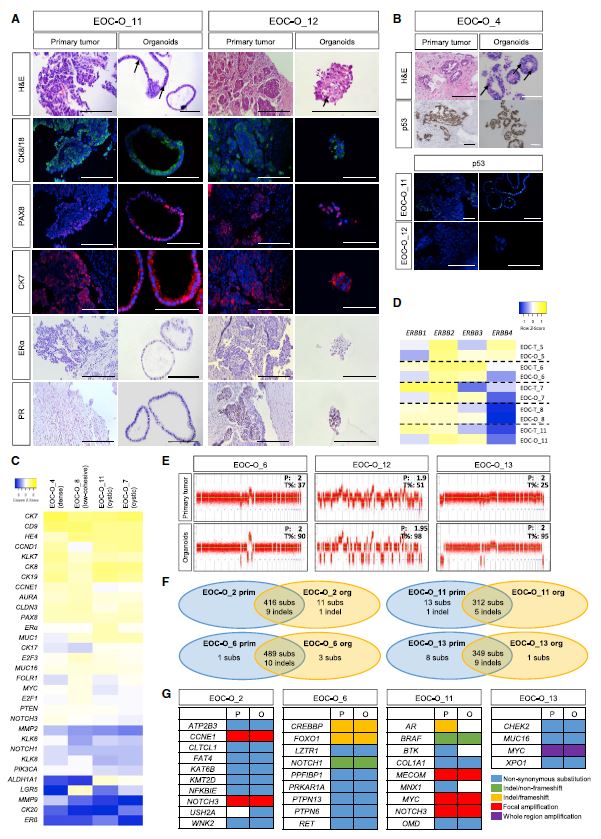 EOC病理生物学的基本机制尚不清楚,治疗效率和患者生存率没有显著改善(Timmermans等人,2018年)。大多数研究都是使用癌细胞系进行的,这些癌细胞系在概括起源肿瘤和EOC性质的组织病理学和分子表型方面表现不佳,因此缺乏临床可翻译性(Lengyel等人,2014年)。患者肿瘤来源的异种移植物生长在免疫缺陷小鼠体内,能更好地模拟原始肿瘤,但其建立效率低、时间长、成本高,在伦理上值得怀疑(Sachs和Clevers,2014)。因此,需要更合适的实验和临床前EOC模型。  将EOC活组织切片(主要为HGSOC;表1)分离并将细胞接种在OC类器官培养基-1(OCOM1;表S1)中,其成分基于先前定义的从子宫内膜和子宫内膜癌中衍生类器官的培养基(Boretto等人,2017、2019)。然而,类器官发育效率低(33%),可膨胀性仅限于1-2代(图S1A和S1B)。因此,我们系统地测试了培养基成分,以促进EOC类器官的建立和生长。降低转化生长因子b(TGFb)途径抑制剂A83-01的浓度,提高烟酰胺的水平,并将RSPO1的来源从细胞系条件培养基改为重组蛋白(culturemedium称为asOCOM2;图S1A和表S1),可增加发育的类器官系的可扩展性(至3–7代;数据未显示),但不能提高形成效率(图S1A和S1B)。进一步修改培养基,包括(1)省略碱性成纤维细胞生长因子(bFGF)和FGF10,(2)添加胰岛素样生长因子1(IGF1)和肝细胞生长因子(HGF)(已知可刺激OC细胞系的生长)(Aune等人,2011)和(3)减少p38丝裂原活化蛋白激酶抑制剂(p38i)SB203580(OCOM3;图S1A和表S1),显示有利于从其他癌症类型(如子宫内膜癌和乳腺癌)建立类器官(Boretto等人,2019;Sachs等人,2018),提高形成效率(图S1A和S1B),但不会进一步增加可扩展性(未显示数据)。据报道,TGFa可诱导癌性OSE中的细胞增殖(Sheng等人,2010年),但并未促进类器官生长的启动(数据未显示),而发现RSPO1是必需的(图S1B;与Kopper等人,2019年和Hill等人,2018年相比)。最后,我们发现添加NRG1(OCOM4;图S1A和表S1)显著增加了形成的类有机物的数量(图S1C),从而独立地(事先不知道)证实并定量支持Kopper等人(2019)最近的发现。NRG1的这种有益作用也与先前的研究一致,这些研究显示NRG1在OC肿瘤和细胞系中具有潜在的(旁分泌)生长刺激作用(Gilmour等人,2002年;Sheng等人,2010年)。我们进一步放大了NRG1的作用,发现类器官培养物中增殖(Ki67+)细胞的数量以及类器官的大小显著增加(图S1D)。综上所述,通过彻底探索多种培养基成分,我们最终确定了一种培养基(OCOM4),该培养基可显著提高EOC类有机物形成效率(从33%提高到56%;图S1A)。有趣的是,添加NRG1也增加了EOC衍生类有机物的可传代性(图S1E)。尽管OCOM4中肿瘤接种时形成的类有机物数量(第0代[P0])并不低于Kopper等人(2019)中使用的培养基(图S1F;“Kopper”培养基,见表S1),但总体类有机物衍生效率相对于患者总数仍然较低(详细比较,见表S2)。讨论中描述了可能的原因。值得注意的是,新鲜获得的活检组织和冷冻保存的活检组织之间的类器官形成效率没有显著差异(图S1G和表1),因此强调了在类器官建立之前储存临床样本的可能性(如一些其他癌症所述,特别是小鼠异种移植和人类乳腺肿瘤;Walsh等人,2016)。此外,类器官可从未接受化疗的患者(通过初次去瘤手术获得)和接受化疗的患者(通过先前新辅助化疗后的间隔去瘤手术获得)的EOC活检中获得(图S1G)。  总之,我们从患者获得的EOC中建立了类器官细胞系,以捕获疾病和患者的肿瘤多样性和特征,从而为现有的EOC类器官细胞库添加了新的细胞系,这对于深入了解癌症的病因、发病机制、异质性和化疗耐药性,以及确定新的治疗靶点和筛选新药至关重要,最好是以患者定制的方式(也在Maru和Hippo,2019年进行了综述)。应该承认,EOCorganoid派生协议仍然值得进一步改进。EOC类器官平台作为一种实验和临床前研究模型具有强大的潜力,特别是作为恢复NRG1/ERBB研究inOC的推动力,并可能最终识别反应预测性生物标志物,协助临床决策,并提供个性化治疗选择,特别是对于标准临床路径已经用尽的患者。 Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models Ovarian cancer (OC) represents the most dismal gynecological cancer. Pathobiology is poorly understood, mainly due to lack of appropriate study models. Organoids, defined as self-developing three-dimensional in vitro reconstructions of tissues, provide powerful tools to model human diseases. Here, we established organoid cultures from patient-derived OC, in particular from the most prevalent high-grade serous OC (HGSOC). Testing multiple culture medium components identified neuregulin-1 (NRG1) as key factor in maximizing OC organoid development and growth, although overall derivation efficiency remained moderate (36% for HGSOC patients, 44% for all patients together). Established organoid lines showed patient tumor-dependent morphology and disease characteristics, and recapitulated the parent tumor’s marker expression and mutational landscape. Moreover, the organoids displayed tumor-specific sensitivity to clinical HGSOCchemotherapeutic drugs. Patient-derivedOCorganoids provide powerful tools for the study of the cancer’s pathobiology (such as importance of the NRG1/ERBB pathway) as well as advanced preclinical tools for (personalized) drug screening and discovery. Ovarian cancer (OC) is the most lethal gynecological cancer. In more than 80% of patients, the disease is not discovered until advanced stage and metastasis (Narod, 2016). After primary debulking surgery and adjuvant chemotherapy, 70%–80% of the patients show tumor relapse with increasing chemoresistance (Pignata et al., 2017). Most OC cases display an epithelial phenotype (epithelial OC [EOC]), with 75% of the patients diagnosed with high-grade serous OC (HGSOC) of FIGO stage III or IV (i.e., showing extensive metastatic spread) (Jelovac and Armstrong, 2011). HGSOC causes up to 80% of the mortality among OC patients, and thus represents the most outstanding clinical challenge in gynecological oncology. Etiology and site of origin of EOC, whether it is ovarian surface epithelium (OSE) or fallopian tube epithelium (FTE) (or both), are still under intense debate (Kim et al., 2018). Mechanisms underlying EOC pathobiology are poorly understood, and therapeutic efficiency and patient survival are not significantly improving (Timmermans et al., 2018). Most studies have been done using cancer cell lines that perform poorly in recapitulating histopathological and molecular phenotype of the tumor of origin and of EOC nature in general, thereby lacking clinical translatability (Lengyel et al., 2014). Patient tumor-derived xenografts, growing in immune-deficient mice, better mimic the original tumor but their establishment is inefficient, lengthy, and costly, and is ethically questionable (Sachs and Clevers, 2014). Therefore, more appropriate experimental and preclinical EOC models are needed. EOC biopsies (predominantly HGSOC; Table 1) were dissociated and cells seeded in OC organoid culture medium- 1 (OCOM1; Table S1), the composition of which was based on the medium previously defined to derive organoids from endometrium and endometrial cancer (Boretto et al., 2017, 2019). However, organoid development efficiency was low (33%) and expandability was limited to 1–2 passages (Figures S1A and S1B). Therefore, we systematically tested culture medium components to improve EOC organoid establishment and growth. Reducing the concentration of the transforming growth factor b (TGFb) pathway inhibitor A83-01, raising the level of nicotinamide, and changing the source of RSPO1 from cell line-conditioned medium to recombinant protein (culturemedium referred to asOCOM2; Figure S1AandTable S1) increased the expandability of developed organoid lines (to 3–7 passages; data not shown) but did not improve formation efficiency (Figures S1A and S1B). Further modification of the medium involving (1) omission of basic fibroblast growth factor (bFGF) and FGF10, (2) addition of insulin-like growth factor 1 (IGF1) and hepatocyte growth factor (HGF), known to stimulate growth ofOC cell lines (Aune et al., 2011), and (3) reduction of the p38 mitogen-activated protein kinase inhibitor (p38i) SB203580 (OCOM3; Figure S1A and Table S1), shown to be beneficial for establishing organoids from other cancer types such as endometrial and breast cancer (Boretto et al., 2019; Sachs et al., 2018), improved formation efficiency (Figure S1A and S1B) but did not further increase expandability (data not shown). TGFa, reported to induce cell proliferation in cancerous OSE (Sheng et al., 2010), did not advance organoid growth initiation (data not shown), while RSPO1 was found to be essential (Figure S1B; comparable with Kopper et al., 2019 and Hill et al., 2018). Finally, we found that addition of NRG1 (OCOM4; Figure S1A and Table S1) significantly increased the number of organoids formed (Figure S1C), thereby independently (without prior knowledge) confirming, and in addition quantitatively supporting, the recent finding by Kopper et al. (2019). This beneficial effect of NRG1 is also in line with previous studies showing a potential (paracrine) growthstimulatory effect of NRG1 in OC tumors and cell lines (Gilmour et al., 2002; Sheng et al., 2010). We further zoomed in on the effect of NRG1 and found a significant increase in the number of proliferating (Ki67+) cells in the organoid cultures as well as of the size of the organoids (Figure S1D). Taken together, by thoroughly probing multiple medium components, we eventually defined a culture medium (OCOM4) that strongly enhanced the EOC organoid formation efficiency (from 33% to 56%; Figure S1A). Interestingly, addition of NRG1 also increased the passageability of the EOC-derived organoids (Figure S1E). Although the number of organoids formed at tumor seeding (passage 0 [P0]) in OCOM4 was not inferior to the culture medium used in Kopper et al. (2019) (Figure S1F; ‘‘Kopper’’medium, see Table S1), overall organoid derivation efficiency over total number of patients remained lower (for a detailed comparison, see Table S2). Possible reasons are described in the Discussion. Of note, organoid formation efficiency did not significantly differ between freshly obtained and cryopreserved biopsies (Figure S1G and Table 1), thereby underscoring the possibility to store clinical samples pending organoid establishment (as described for some other cancers, in particular mouse xenograft and human breast tumors; Walsh et al., 2016). Furthermore, organoids could be derived from EOC biopsies of both chemo-naive patients (obtained by primary debulking surgery) and chemotherapy-treated patients (obtained by interval debulking surgery after prior neoadjuvant chemotherapy) (Figure S1G). In summary, we established organoid lines from patientderived EOC that capture disease and patients’ tumor diversity and hallmarks, thereby adding new lines to the existing EOC organoid repertoire, which is essential for gaining deeper insight into the cancer’s etiology, pathogenesis, heterogeneity, and chemoresistance, and for identifying newtherapeutic targets and screening newdrugs, preferably in a patient-tailored manner (also reviewed in Maru and Hippo, 2019). It should be acknowledged that the EOCorganoid derivation protocol still deserves further efforts for improvement. The EOC organoid platform has strong potential as an experimental and preclinical research model, and in particular as impetus to revive NRG1/ERBB research inOC, and may eventually identify response-predictive biomarkers, assist in clinical decision making, and provide personalized therapeutic options, particularly for patients in whom standard clinical routes have been exhausted. |
