Research Focus |
|
患者源性类有机物识别KRAS突变结肠癌治疗诱导的脆弱性的药物再利用筛选 2024-04-08 20:26:02 浏览次数:586 | |
| 患者源性类有机物识别KRAS突变结肠癌治疗诱导的脆弱性的药物再利用筛选 来源:仪方生物 www.yeslab.com 患者衍生类有机物(PDOs)被广泛认为是开发新的抗肿瘤治疗药物的筛选平台。在这里,我们使用药物重组文库来筛选结直肠癌(CRC)的PDO,以确定治疗诱导表型中隐藏的弱点。利用一个基于显微镜的屏幕,准确地评分药物诱导的细胞杀伤,我们已经测试了414个假定的抗癌药物的能力,转换为细胞毒性EGFRi/MEKi诱导的细胞抑制表型。大多数已验证的hits(9/37)是临床肿瘤常用的微管靶向药物,如紫杉烷和长春花生物碱。其中一种药物,长春瑞滨,在25个不同的CRC PDO组中始终有效,与RAS突变状态无关。与长春瑞滨单独使用不同,其与EGFR/MEK抑制联合使用可在细胞周期的各个阶段诱导细胞凋亡,并在体内表现出耐受性和有效的抗肿瘤活性,为治疗转移性RAS突变结直肠癌患者的临床试验奠定了基础。 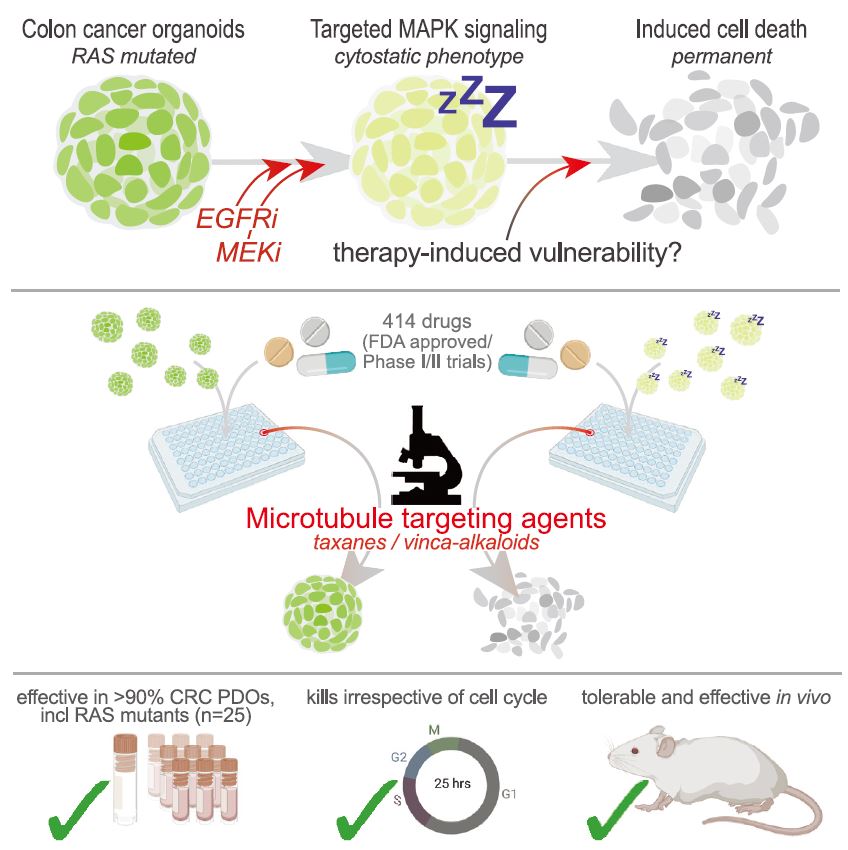 EGFR-RAS-MAPK通路是结直肠癌(CRC)中最常见的去调节信号级联通路之一,但靶向KRAS或BRAF突变的CRC肿瘤的有效治疗策略已被证明是有问题的。1,2尽管癌基因驱动突变促进KRAS3和BRAF的组成性激活,4最有希望的临床结果是通过抑制丝裂原活化蛋白激酶(MAPK)通路与上游表皮生长因子受体(EGFR)结合。5–8然而,特别是对于RAS突变的CRC,EGFR-RAS-MAPK通路的这种组合靶向最多可导致疾病稳定,9–11突出表明诱导细胞毒性在RAS突变的肿瘤中仍然存在问题。仅抑制下游MAPK通路活性。  过去几年中,2D细胞系上的基因筛查记录了RAS突变癌中的特定脆弱性。12,13然而,这些方法在识别靶向RAS驱动的肿瘤生长的可行治疗策略方面的成功受到限制。1,14相反,下游MAPK通路信号的有效抑制在小鼠中显示出显著的抗肿瘤活性。15不幸的是,靶向抑制剂对EGFR-MAPK通路有效所需的剂量总是伴随着较差的耐受性。1因此,与其提高EGFR-MAPK通路抑制的有效性,使其超过与毒性相关的水平,探索其他靶点的潜力很大。一种可能的方法是通过联合靶向治疗诱导的易损性,将EGFR-MAPK通路抑制的细胞抑制作用转化为细胞毒性。事实上,利用基因敲除或药物抑制BCL-xL来共同靶向凋亡途径已被报道为一种有效的策略。16,17然而,急性血小板减少症是已知的直接抑制BCL-xL的剂量限制性毒性。18,19结直肠癌患者源性类器官(PDO)在体外是稳健的模型,保留了体内肿瘤的组织病理学特征。 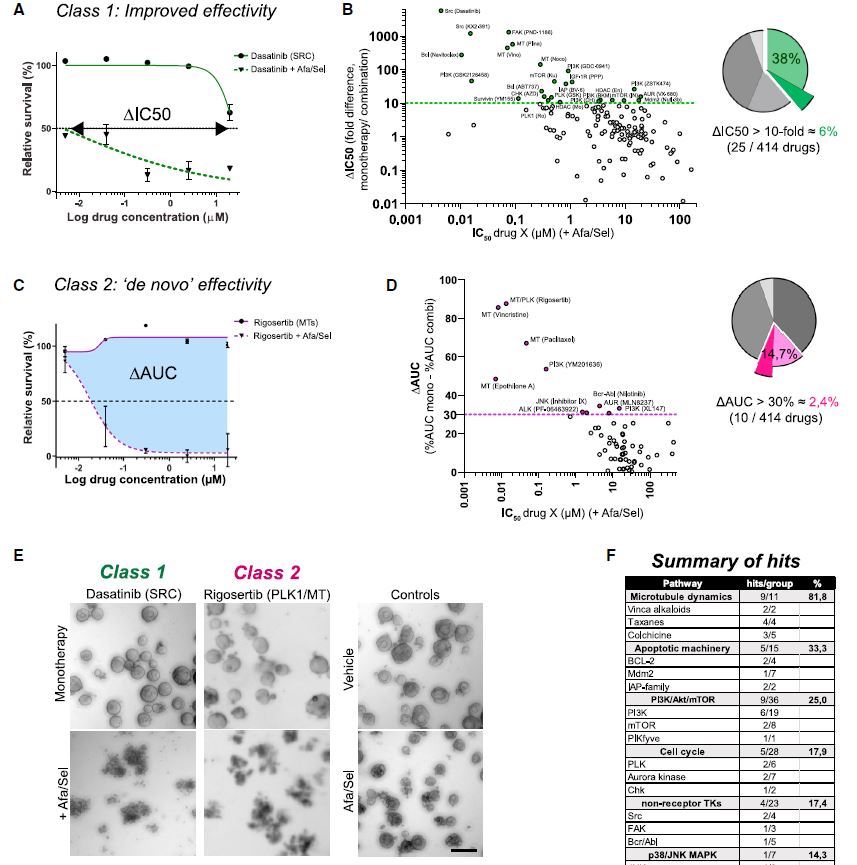 筛选对人类肿瘤类器官具有抗癌效果的候选药物通常使用高通量代谢读数(由Wensink等人25综述)。尽管有助于对药物敏感性和can-cer突变之间的模式进行评分,但此类分析中的一个重要警告是缺乏对细胞抑制和细胞毒性药物效应的区分。17,32说明性的是通过临床相关的pan-HER和MEK抑制剂组合靶向抑制KRAS突变体肿瘤类器官(CRC-PDO)中的EGFR-RAS-MAPK通路。虽然药物诱导的效应与代谢率的显著降低相适应,但显微镜分析显示所需细胞死亡的轻微诱导(图1A)。不能用常用的高通量检测方法准确检测细胞死亡,不利于在KRAS突变肿瘤中发现新的治疗弱点,从而增强EGFR和MEK联合抑制的抗癌效果。 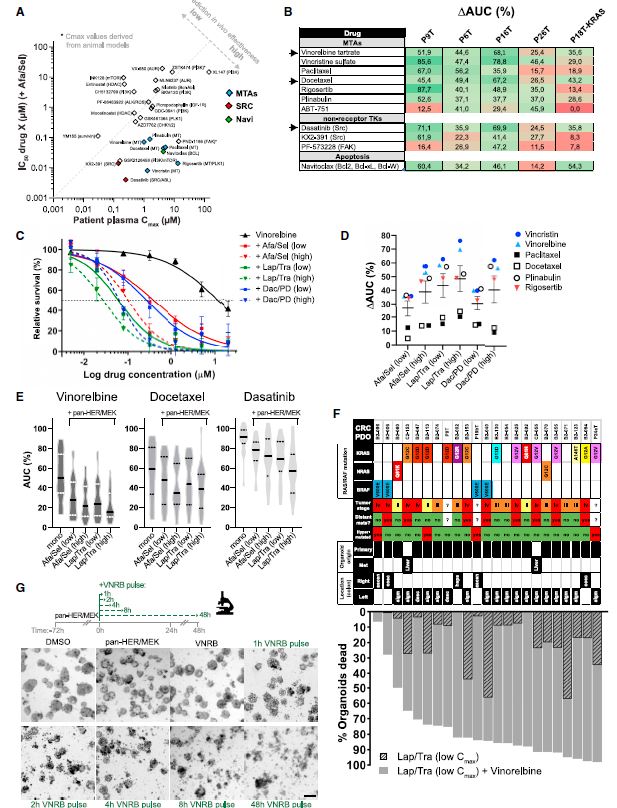 自动化显微镜和图像分析的最新进展使复杂的策略能够在3D模型系统中对药物诱导的phe类型进行评分。为了筛选PDO中药物诱导的细胞毒性,我们开发了一种半自动化图像分析管道,该管道以384孔板中的器官培养物的广域成像为中心。将类器官培养的荧光图像分割成单个目标,并分析循环度和尺寸等参数。特别是,圆形评分作为药物诱导细胞毒性的可靠读数,因为细胞死亡影响上皮完整性并降低正常近囊性形态的圆形性。健康培养物内的所有类有机物C评分与100%药物诱导细胞毒性之间的比较确实揭示了这些类有机物群体的C评分分布之间的最小重叠。 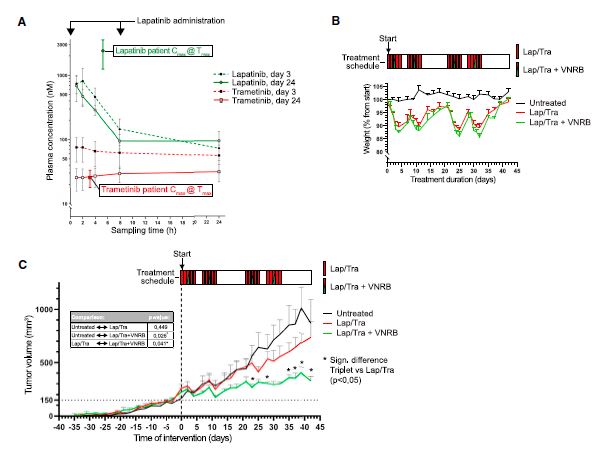 我们在这里展示了VNRB-pan-HER/MEK抑制在20个以上的CRC PDO中的一致有效性,这与RAS突变状态无关。此外,如果下游MAPK信号通路被有效抑制,当EGFR/MEK抑制剂被不同但机制相同的药物替代时,抗肿瘤作用仍然不受影响。考虑到负担得起的医疗保健,我们成功地将VNRB与靶向抑制剂Lap(pan-HER)和binimetinib(MEK)结合起来,这两个专利家族相对于其竞争对手而言都是首次过期的。已开始进行一项I/II期临床试验,以测试这种三联疗法对转移性RAS突变型CRC患者的有益效果(RASTRIC-EudraCT:2019-004987-23)。最近,新的EGFR-RAS-MAPK通路抑制剂已经被开发出来,例如通过特异性靶向突变型KRASG12C或使用针对EGFR的肿瘤特异性抗体。31,59尽管预期肿瘤特异性会得到改善,但我们主张这些新疗法仍然可以通过联合服用VNRB(临床肿瘤学中常用的药物)获得额外的抗肿瘤活性 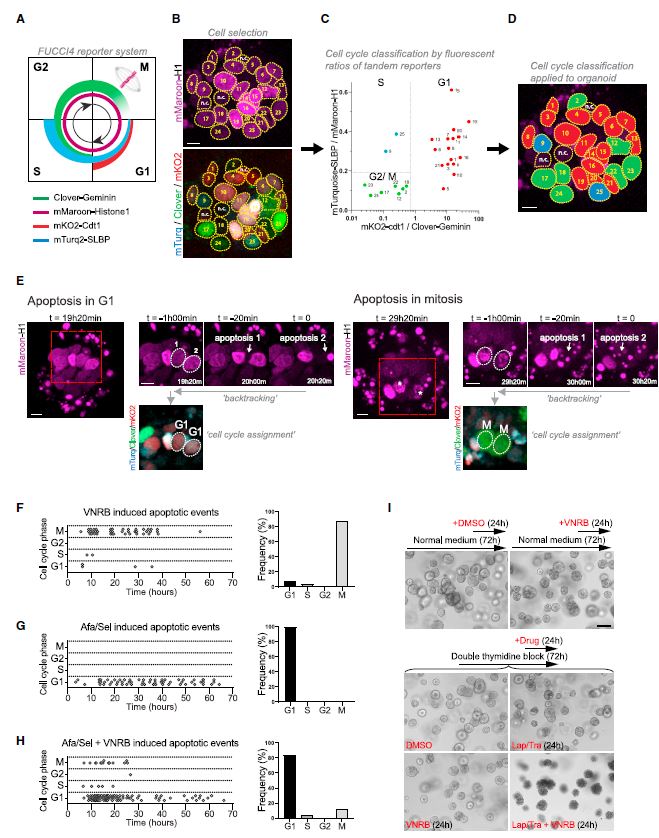 Drug-repurposing screen on patient-derived organoids identifies therapy-induced vulnerability in KRAS-mutant colon cancer Patient-derived organoids are widely heralded as a drug-screening platform to develop new anti-can- cer therapies. Here, we use a drug-repurposing library to screen PDOs of colorectal cancer to identify hidden vulnerabilities within therapy-induced phenotypes. Using a microscopy-based screen that accurately scores drug-induced cell killing, we have tested 414 putative anti-cancer drugs for their ability to switch the EGFRi/MEKi-induced cytostatic phenotype toward cytotoxicity. A majority of validated hits are micro- tubule-targeting agents that are commonly used in clinical oncology, such as taxanes and vinca-alkaloids. One of these drugs, vinorelbine, is consistently effective across a panel of >25 different CRC PDOs, indepen- dent of RAS mutational status. Unlike vinorelbine alone, its combination with EGFR/MEK inhibition induces apoptosis at all stages of the cell cycle and shows tolerability and effective anti-tumor activity in vivo, setting the basis for a clinical trial to treat patients with metastatic RAS-mutant CRC. The EGFR-RAS-MAPK pathway is among the most frequently deregulated signaling cascades in colorectal cancer , but effective therapeutic strategies to target KRAS or BRAF-mutant CRC tumors have proven to be problematic.1,2 Although onco-genic driver mutations prompt the constitutive activation of KRAS3 and BRAF,4 the most promising clinical results encom-pass inhibition of the mitogen-activated protein kinase pathway in combination with the upstream epidermal growth factor receptor .5–8 However, in particular for RAS-mutant CRC, such combinatorial targeting of the EGFR-RAS-MAPK pathway results in disease stabilization at best,9–11 highlighting that induction of cytotoxicity remains problematic in RAS-mutant tumors with sole suppression of downstream MAPK pathway activity. Over the past years, genetic screens on 2D cell lines have documented specific vulnerabilities in RAS-mutant cancers.12,13 However, the success of these approaches in identifying viable therapeutic strategies that target RAS-driven tumor growth has been limited.1,14 In contrast, effective inhibition of downstream MAPK pathway signaling demonstrated significant anti-tumor activity in mice.15 Unfortunately, the required doses of targeted inhibitors to be effective against the EGFR-MAPK pathway are invariably accompanied by poor tolerability.1 Therefore, rather than improving effectivity of EGFR-MAPK pathway inhibition beyond levels that are associated with toxicity, there is high po-tential in exploring additional targets. One possibility is to convert the cytostatic effect of EGFR-MAPK pathway suppres-sion into cytotoxicity by co-targeting therapy-induced vulnera-bilities. Indeed, co-targeting the apoptotic pathway using ge-netic depletion or pharmacological inhibition of BCL-xL has been reported as a valid strategy.16,17 However, acute thrombo-cytopenia is a known dose-limiting toxicity regarding direct BCL-xL inhibition.18,19 Patient-derived organoids of CRC are robust in vitro models that retain the histopathological features of in vivo tu-mors.20,21 Screening drug candidates for anti-cancer effectivity on human tumor organoids is commonly performed using high-throughput metabolic readouts . Although informative to score patterns between drug sensitivity and can-cer mutations, an important caveat in such assays is the lack of discrimination between cytostatic and cytotoxic drug ef-fects.17,32 Illustrative is targeted inhibition of the EGFR-RAS-MAPK pathway in KRAS-mutant tumor organoids with a clinically relevant combination of pan-HER and MEK inhibitors. While the drug-induced effects are accompa-nied by significant reductions in metabolic rates, microscopic analysis shows minor induction of the desired cell death (Fig-ure 1A). The inability to faithfully detect cell death with commonly used high-throughput assays is detrimental to iden-tifying new therapeutic vulnerabilities in KRAS-mutant tumors that enhance anti-cancer effectivity of combined EGFR and MEK inhibition. Recent advances in automated microscopy and image anal-ysis enable sophisticated strategies to score drug-induced phe-notypes in 3D model systems.29,31–33 To screen for drug-induced cytotoxicity in PDOs, we developed a semi-automated image analysis pipeline centered on wide-field imaging of orga-noid cultures in 384-well plates . Fluorescent images of organoid cultures were segmented into single objects and analyzed for parameters such as circu-larity and size. In particular, the circularity score served as a reliable readout for drug-induced cytotoxicity, as cell death af-fects epithelial integrity and reduces circularity of the normally near-cystic morphology. Comparison between all the organoids C scores within a healthy culture versus 100% drug-induced cytotoxicity indeed revealed a minimal overlap between the C-score distributions of these organoid populations. |
