Research Focus |
|
胆管癌患者来源类器官的建立及药物筛选 2024-04-11 10:25:46 浏览次数:763 | |
| 胆管癌患者来源类器官的建立及药物筛选 来源:仪方生物 www.yeslab.com 胆道癌(BTCs)是最具侵袭性的恶性肿瘤之一,预后较差。在这里,我们成功地建立了来源于肝内胆管癌、癌症和瓦特尔安瓿神经内分泌癌的类器官系。这些来源于BTCs的类器官稳定培养了>1年,并与原发性肿瘤中明显的组织病理学、基因表达和遗传变化密切相关。类器官的基因表达谱显示,SOX2可能是BTC患者的潜在预后生物标志物。我们筛选了一个由临床上使用的药物组成的化合物库,以确定其抑制BTCs衍生类器官的能力,发现抗真菌药物阿莫罗芬和芬替康唑显著抑制BTCs来源类器官的生长,对正常胆管上皮细胞的毒性最小。患者来源的类器官可能是阐明分子发病机制和发现难治性癌症生物标志物和治疗药物的有力研究工具。 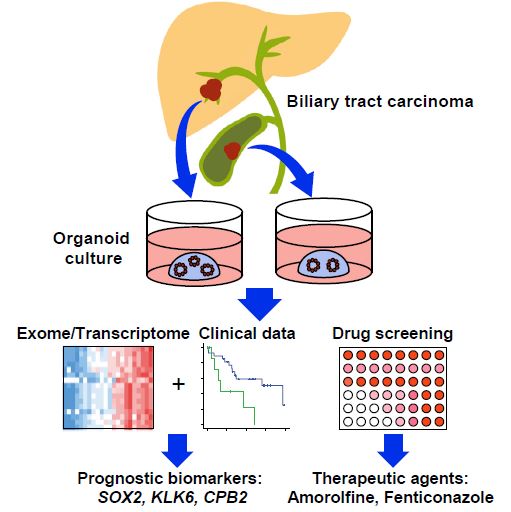 胆管癌(BTCs)是发生在肝内胆管和胆总管远端的沃特壶腹之间区域的上皮性恶性肿瘤。BTC可分为肝内胆管癌(IHCC)、肝外胆管癌(EHCC)和胆囊癌症(GBC)。BTC患者的数量在全球范围内明显增加。尽管手术切除是BTCs患者的唯一治疗方法,但大多数病例都是在晚期诊断的,结果不佳。无法手术的BTCs患者通常接受化疗方案,包括吉西他滨,但其效果有限,患者的5年生存率非常低。  LGR5是WNT信号通路的一个成员,已被鉴定为小肠、结肠、胃、肝脏和胰腺中干细胞的新分子标记物(Barker等人,2010;Huch等人,2013a,2013b;Sato等人,20092011a,2011b)。被称为类器官培养的新开发的3D培养系统允许LGR5+干细胞长期扩增成具有与原始组织相似特性的囊状结构(类器官)。这种类型的3D培养物使用无血清培养基,该培养基仅包括定义的因子,如R-反应蛋白1(Rspo1)、表皮生长因子(EGF)和诺金。Rspo1已被鉴定为LGR5的配体和增强WNT信号通路的重要因子(Carmon等人,2011;de Lau等人,2011)。使用这种新的3D培养系统,已经建立了人类结肠癌、前列腺癌、胰腺癌和肝癌的类器官模型(Boj等人,2015;Broutier等人,2017;Gao等人,2014;Matano等人,2015年;Sato等人,2011a)。我们还报道了DNA甲基化的抑制抑制了肠道肿瘤类器官的生长,并且当IHCC类器官被诱导分化为肝细胞时,其恶性潜能降低(Saito等人,20162018)。  我们成功地建立了6个癌症类器官系,并稳定培养了1年以上。这些病例的病理诊断为IHCC、胰腺导管腺癌(PDA)、GBC和壶腹神经内分泌癌(NEC)(表S1)。如前所述(Ojima等人,2010),使用来源于IHCC患者(1T)的异种移植物组织建立类器官系1。使用从诊断为患有下胆管癌症(3T)的患者获得的癌症组织建立类器官系2,但手术后的病理诊断为PDA。使用分别从患有IHCC(9T和24T)、GBC(19T)和Vater安瓿NEC(36T)的患者获得的癌症组织建立类器官系3-6(表S1)。经过类器官培养的沃特壶腹(36T)的IHCC(9T)、PDA(3T)、GBC(19T)和NEC的手术切除组织标本的宏观特征分别如图1A、2A、2D和2G所示。这些癌症类器官系允许细胞在冷冻和解冻后长期体外扩增和恢复。如表S1所示,我们试图使用从BTC患者获得的总共18个手术切除的组织标本来建立类器官,但18个类器官中的12个长期培养不成功。当我们分别使用6个、5个和2个IHCC、GBC和PDA的组织标本时,建立IHCC、GB和PDA类器官的成功率分别为50%(3/6)、20%(1/5)和50%(1/2)。我们还使用1个组织样本(100%,1/1)建立了源自罕见癌症的类器官,即瓦特尔安瓿的NEC。从几个胆管癌(BDC)样本中建立癌症类器官的尝试没有成功。 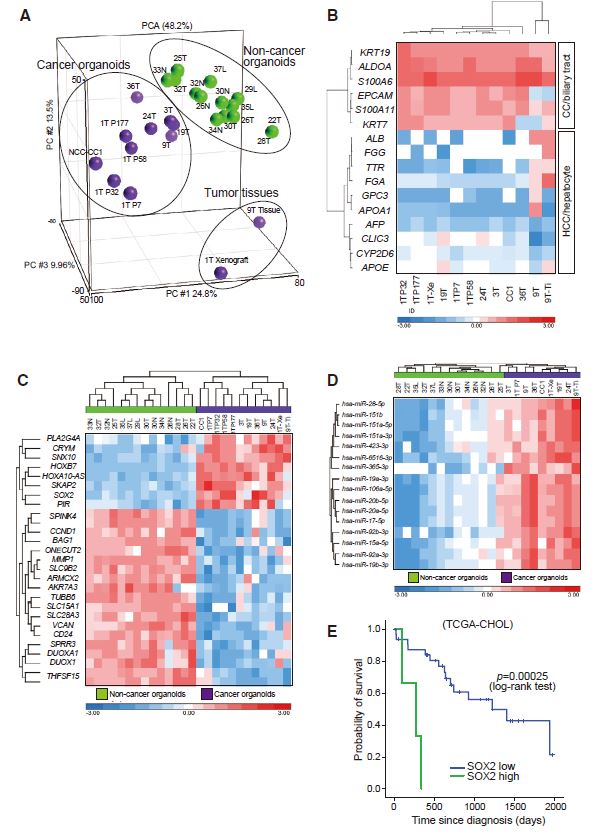 目前,包括吉西他滨在内的化疗方案是无法手术的BTCs患者的标准治疗方法。然而,它们的作用是有限的,患者的5年生存率非常低。严重的不良反应也是这些抗癌药物中的一个主要问题。我们的研究结果表明,坚果蛋白-3a,一种MDM2抑制剂,可能是一种潜在的治疗携带野生型TP53的难治性癌症的药物。我们还确定KLK6和CPB2为潜在的生物标志物,可预测EGFR抑制剂埃洛替尼的疗效和药物敏感性。 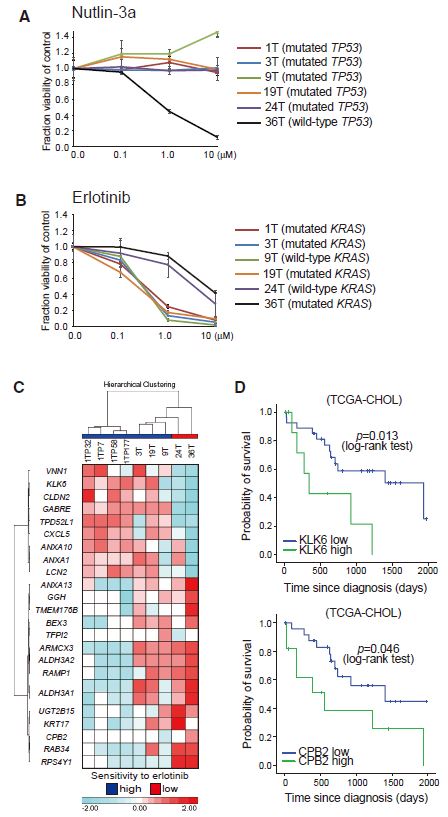 在药物重新定位策略中,从已经在临床使用的药物库中筛选具有临床有益药理活性的化合物,目的是为它们开发新的适应症。这种策略的优点是降低了对人类产生意外不良反应的风险,因为这些药物的安全性已经在人类身上得到了很好的表征。在这里,我们发现抗真菌药物阿莫洛芬和芬替康唑显著抑制BTCs衍生的类器官的生长,对正常胆管上皮细胞的毒性较小。这些药物可以有效预防和治疗BTCs,不良反应最小。需要进行包括临床试验在内的进一步研究,以检查这些药物在预防BTCs和治疗受影响患者方面的潜在临床应用。 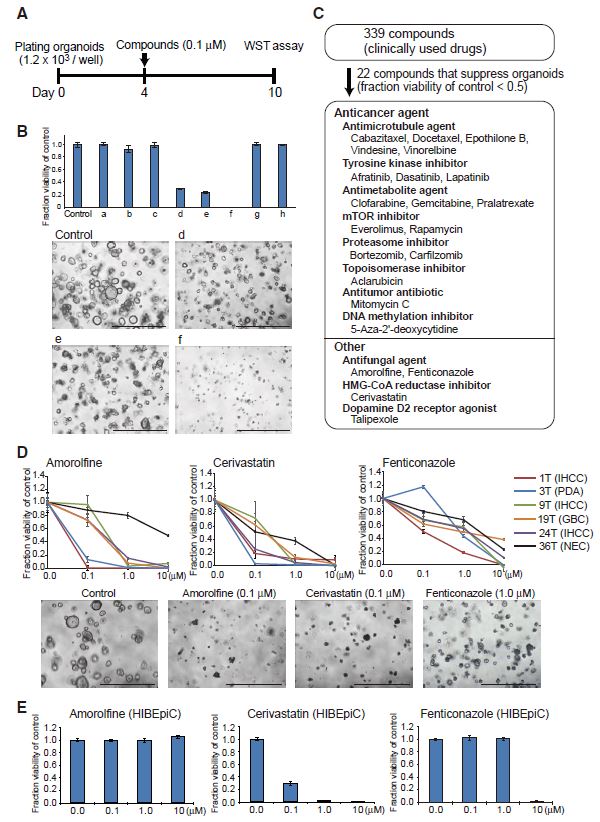 Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma Biliary tract carcinomas (BTCs) are among the most aggressive malignancies and have a poor prognosis. Here, we successfully established organoid lines derived from intrahepatic cholangiocarcinoma, gallbladder cancer, and neuroendocrine carcinoma of the ampulla of Vater. These organoids derived from BTCs were cultured stably for >1 year and closely recapitulated the histopathology, gene expression, and genetic alterations evident in the primary tumors. Gene expression profiling of the organoids revealed that SOX2 could be a potential prognostic biomarker for patients with BTC. We screened a compound library consisting of drugs used clinically for their ability to suppress organoids derived from BTCs and found that the antifungal drugs amorolfine and fenticonazole significantly suppressed the growth of organoids derived from BTCs with minimal toxicity to normal biliary epithelial cells. Patient-derived organoids may be a powerful research tool for the clarification of molecular pathogenesis and the discovery of biomarkers and therapeutic drugs for refractory cancers. Biliary tract carcinomas (BTCs) are epithelial malignancies arising in the region between the intrahepatic bile ducts and the ampulla of Vater at the distal end of the common bile duct. BTCs can be divided into intrahepatic cholangiocarcinoma (IHCC), extrahepatic cholangiocarcinoma (EHCC), and gallbladder cancer (GBC). The number of BTC patients is apparently increasing worldwide. Although surgical resection is the only curative treatment for patients with BTCs, most cases are diagnosed at an advanced stage and have poor outcomes. Patients with inoperable BTCs generally receive chemotherapy regimens, including gemcitabine, but their effects are limited, and the 5-year survival rates of patients are very low. LGR5, a member of the WNT signaling pathway, has been identified as a new molecular marker of stem cells in the small intestine, colon, stomach, liver, and pancreas (Barker et al., 2010; Huch et al., 2013a, 2013b; Sato et al., 2009, 2011a, 2011b). The newly developed 3D culture system known as organoid culture allows long-term expansion of LGR5+ stem cells into cyst-like structures (organoids) with properties resembling those of the original tissues. This type of 3D culture uses a serum-free medium that includes only defined factors such as R-spondin 1 (Rspo1), epidermal growth factor (EGF), and Noggin. Rspo1 has been identified as a ligand for LGR5 and an essential factor for the potentiation of the WNT signaling pathway (Carmon et al., 2011; de Lau et al., 2011). Using this new 3D culture system, organoid models of human colon, prostate, pancreatic, and liver cancers have been established (Boj et al., 2015; Broutier et al., 2017; Gao et al., 2014; Matano et al., 2015; Sato et al., 2011a). Wehave also reported that the inhibition of DNAmethylation suppresses the growth of intestinal tumor organoids, and that when IHCC organoids are induced to differentiate to hepatocytes, their malignant potential is reduced (Saito et al., 2016, 2018). We were successful in establishing 6 cancer organoid lines that were cultured stably for >1 year. The pathological diagnoses of these cases were IHCC, pancreatic ductal adenocarcinoma (PDA), GBC, and neuroendocrine carcinoma (NEC) of the ampulla of Vater (Table S1). Organoid line 1 was established using xenograft tissue derived from an IHCC patient (1T), as described previously (Ojima et al., 2010). Organoid line 2 was established using cancer tissue obtained from a patient diagnosed as having lower bile duct cancer (3T), but the pathological diagnosis after surgical operation was PDA. Organoid lines 3–6 were established using cancer tissues obtained from patients with IHCCs (9T and 24T), GBC (19T), and NEC of the ampulla of Vater (36T), respectively (Table S1). The macroscopic features of the surgically resected tissue specimens of IHCC (9T), PDA (3T), GBC (19T), and NEC of the ampulla of Vater (36T) subjected to organoid culture are shown in Figures 1A, 2A, 2D, and 2G, respectively. These cancer organoid lines allowed long-term in vitro expansion and recovery of the cells after freezing and thawing. As shown in Table S1, we tried to establish organoids using a total of 18 surgically resected tissue specimens obtained from BTC patients, but long-term culture was unsuccessful for 12 of the 18 organoids. As we used 6, 5, and 2 tissue specimens of IHCC, GBC, and PDA, respectively, the success rates for establishment of IHCC, GBC, and PDA organoids were 50% (3/6), 20% (1/5), and 50% (1/2), respectively. We also established organoids derived from a rare cancer, NEC of the ampulla of Vater, using 1 tissue specimen (100%, 1/1). Attempts to establish cancer organoids from several samples of bile duct carcinoma (BDC) were unsuccessful. At present, chemotherapy regimens that include gemcitabine are the standard treatment for patients with inoperable BTCs. However, their effects are limited, and the 5-year survival rates of patients are very low. Severe adverse effects are also a major problem in these anticancer agents. Our results indicate that nutlin- 3a, an inhibitor of MDM2, could be a potential therapeutic drug for refractory cancers harboring wild-type TP53. We also identified KLK6 and CPB2 as potential biomarkers that are predictive of both outcome and drug sensitivity to the EGFR inhibitor erlotinib. In a drug repositioning strategy, compounds with clinically beneficial pharmacological activity are screened from a library of medicines already in clinical use, with the aim of developing new indications for them. The advantage of this strategy is that the risk of unexpected adverse effects in humans is reduced, because the safety aspects of these drugs have already been well characterized in humans. Here, we found that the antifungal drugs amorolfine and fenticonazole significantly suppressed the growth of organoids derived from BTCs with less toxicity to normal biliary epithelial cells. These drugs could provide effective prevention and treatment of BTCs with minimal adverse effects. Further studies including clinical trials will be necessary to examine the potential clinical application of these drugs for the prevention of BTCs and the treatment of affected patients. |
