Research Focus |
|
胃类器官——研究胃发育的体外模型系统和个性化用药之路 2024-04-11 10:25:46 浏览次数:1130 | |
| 胃类器官——研究胃发育的体外模型系统和个性化用药之路 来源:仪方生物 www.yeslab.com 癌症是人类最常见的第五大恶性肿瘤,也是癌症相关死亡的第三大原因。根据肿瘤分期,内镜或手术切除辅以围手术期化疗是患者唯一的治疗选择。由于晚期临床表现和缺乏可靠的生物标志物,早期检测具有挑战性,总体生存率仍然很低。类器官是在三维培养的细胞聚集体,其生长特性与其来源组织相似。由于其自我更新和增殖能力,类器官可以在培养中长期保持,并在许多情况下以无限的方式扩展。患者来源的类器官(PDO)库作为活的生物库,可以深入分析组织特异性功能、发育和疾病。最近癌症PDO的成功建立为多种转化临床应用开辟了新的前景。在这里,我们回顾了不同的成人干细胞衍生的胃类器官模型系统,并重点介绍了它们的建立、表型和基因型特征以及它们在预测治疗反应中的应用。 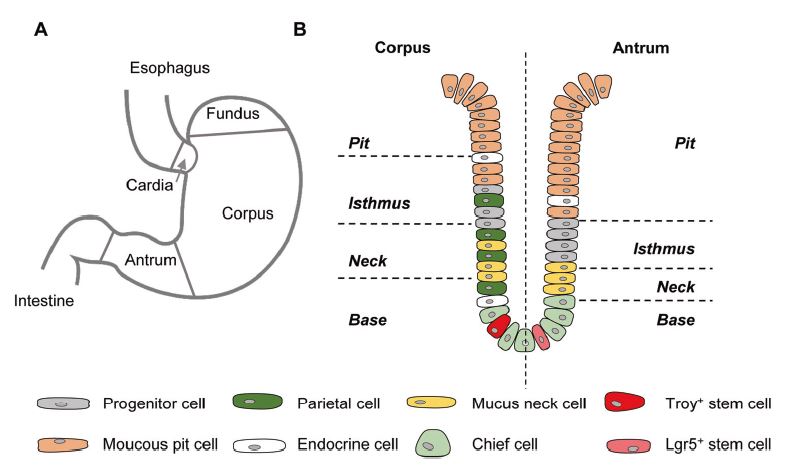 胃——食物储存和消化的器官解剖学、腺体结构和功能胃是一个肌肉器官,在食物储存和吸收中起着重要作用。它由四个主要部分组成:贲门、眼底、体(体)和窦(幽门)(图1A)[1]。胃体是胃分泌酸和消化酶的主要部分。窦在激素和粘液分泌中起着重要作用。鼠胃还具有鳞状上皮衬里的前胃,对食物的储存和机械分解很重要[2-5]。胃与营养物质、毒素和细菌永久接触,它们共同产生有毒环境[6]。为了确保黏膜完整且功能正常,上皮细胞需要不断自我更新。 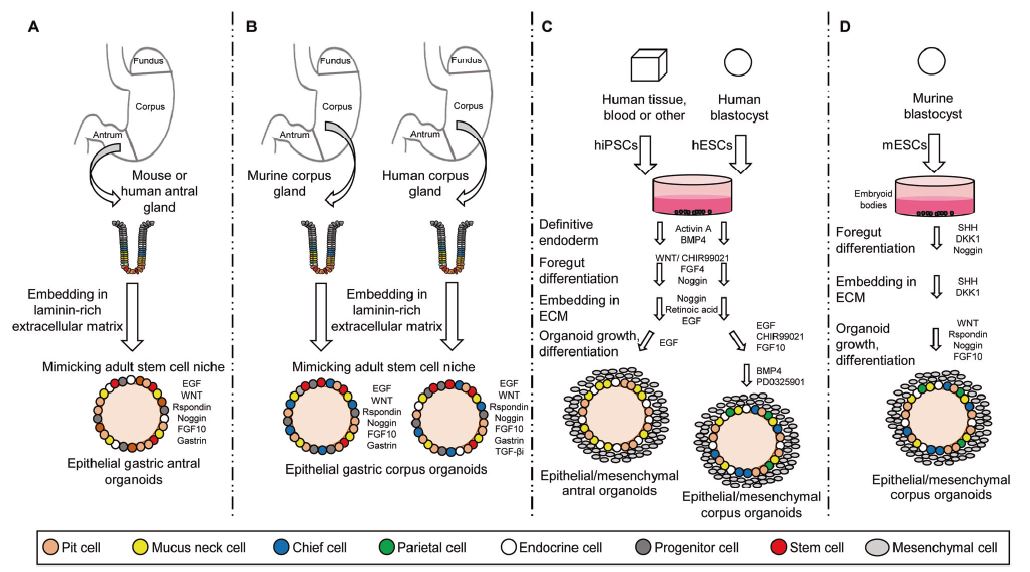 胃黏膜由上皮层组成,上皮层被组织成腺体。从下到上有四个不同的区域:基部、颈部、峡部和凹陷。体上皮呈长腺和短凹坑,其中含有分泌粘液的凹坑和颈部细胞、分泌酸的顶叶细胞、分泌激素的内分泌细胞和分泌消化酶的主细胞(图1B)[7]。Antral腺体较短,并且有一个相对较大的凹陷区域。它们还含有分泌粘液的凹坑和颈部细胞以及内分泌细胞,但位于基底的主要细胞较少,没有顶叶细胞[8]。峡部区域含有增殖细胞,长期以来一直被认为是胃上皮干细胞的家园[9]。正如它们的名字所暗示的,粘液坑细胞只在坑区发现,粘液颈细胞在颈区发现,而主要细胞只位于基部区域。分泌激素的内分泌和分泌酸的顶叶细胞散布在整个腺体中。 胃类器官成体干细胞和多能干细胞允许建立胃类器官胃类器官可以由胃组织衍生的AdSC和PSC启动。这两种系统之间的主要区别在于PSC衍生的类器官培养物中存在间充质细胞。AdCS只能从来源组织中产生特定的细胞,而PSCs本质上具有分化为任何细胞类型的能力。因此,PSC衍生的类器官需要逐步分化方案,该方案将PSC分化为靶组织身份,而AdSC衍生的胃类器官从一开始只需要单一的生长因子富集培养基。因此,将PSC分化为类器官的时间约为30-60天,而AdSC衍生的类器官在7-14天内建立。  从含有Lgr5+干细胞的窦腺建立了第一个鼠成体干细胞来源的胃类器官培养物。该方案是在肠道类器官培养系统的基础上通过添加成纤维细胞生长因子10(FGF10)和胃泌素激素制定的(图2A)[14]。此外,可以观察到主要细胞的标志物(胃蛋白酶原C(PGC))和粘液颈细胞(MUC6)。WNT浓度的降低导致粘液坑和内分泌细胞分化谱系的产生,而没有观察到顶叶细胞[14]。同样的条件后来用于源自Troy+干细胞的鼠体类器官(图2B)[19]。这些类器官表达主要细胞和粘液颈细胞的标志物。在WNT退出后,可以观察到Noggin和FGF10分化的凹坑细胞,但没有内分泌或顶叶细胞。 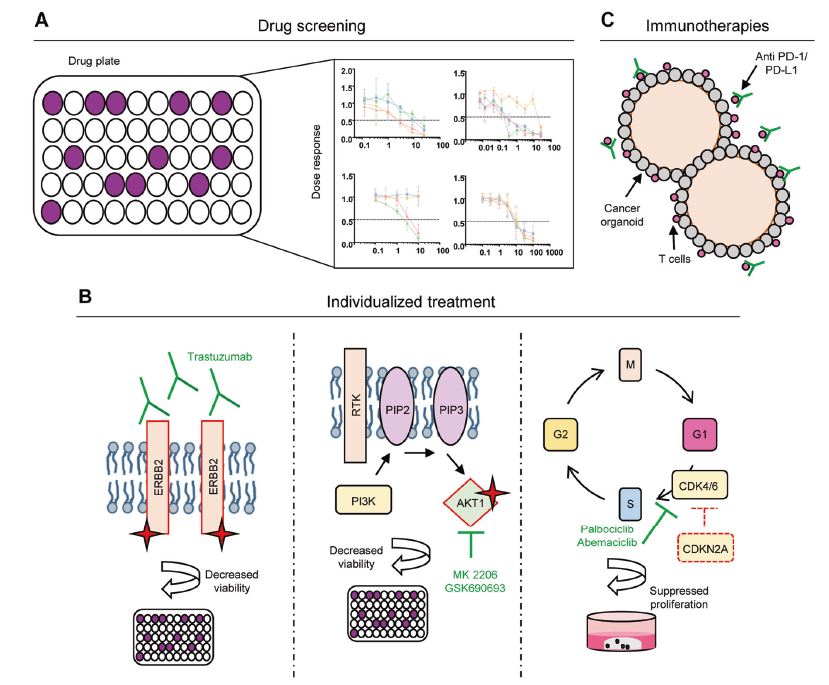 综合来看,第一批数据表明患者来源的类器官在治疗预测中的有用性。根据我们的经验,癌症类器官可以在2-3周内从约50%的患者的活检中确定,其范围可以测试对标准化疗药物的反应。这与开始新辅助化疗前的常规工作(即CT和腹腔镜分期、静脉端口植入)的时间框架相似。在应用进一步的个性化方法之前,姑息治疗环境中的患者通常会根据既定方案接受一线化疗。这通常给了足够的时间来扩展培养物以进行药物筛选,即以Du等人建立的高通量药物筛选的形式,在需要第二/第三线治疗方案的建议之前[117]。因此,在我们看来,未来将有可能将PDO整合到临床决策过程中。值得注意的是,Yan等人报道了一个肿瘤不同肿瘤区域的PDO之间的异质性药物反应[84]。因此,需要在更大的患者队列中进行进一步的联合临床和首次前瞻性研究,以明确PDO在不同治疗环境(即新辅助/姑息治疗)中的作用。此外,对于新的免疫治疗方法,与免疫细胞的共培养系统也需要在肿瘤-巨噬细胞相互作用的体外功能方面进行验证[118]。第一个有希望的数据提示了使用免疫细胞类器官共培养物进行功能测试的可能性,例如检查点抑制疗法(图5C)[119]。 最后,为了进一步引起读者的兴趣,我们想引导读者阅读关于类器官主题不同方面的其他优秀评论[40,100,120–130]。几篇文章综述了癌症以外其他实体肿瘤类器官库的产生[131-136]。 Gastric organoids—an in vitro model system for the study of gastric development and road to personalized medicine Gastric cancer ranks as the fifth most common human malignancy and the third leading cause of cancer related deaths. Depending on tumor stage, endoscopic or surgical resection supported by perioperative chemotherapy is the only curative option for patients. Due to late clinical manifestation and missing reliable biomarkers, early detection is challenging and overall survival remains poor. Organoids are cell aggregates cultured in three-dimensions that grow with similar characteristics as their tissue-of-origin. Due to their self-renewal and proliferative capacity, organoids can be maintained long term in culture and expanded in many cases in an unlimited fashion. Patient-derived organoid (PDO) libraries function as living biobanks, allowing the in depth analysis of tissue specific function, development and disease. The recent successful establishment of gastric cancer PDOs opens up new perspectives for multiple translational clinical applications. Here, we review different adult stem cell derived gastric organoid model systems and focus on their establishment, phenotypic and genotypic characterizations as well as their use in predicting therapy response. The stomach—organ for food storage and digestion Anatomy, gland structure and function The stomach is a muscular organ playing an important role in food storage and digestion. It consists of four main parts: cardia, fundus, corpus (body), and antrum (pylorus) (Fig. 1A) [1]. The corpus is the main part of the stomach secreting acid and digestive enzymes. The antrum plays an important role in hormone and mucus secretion. The murine stomach additionally possesses a squamous-epithelium lined forestomach important for storage and mechanical dissociation of food [2–5]. The stomach is in permanent contact to nutrients, toxins and bacteria that altogether generate a toxic environment [6]. To ensure an intact and functional mucosa, a continuous self-renewal of the epithelium is required. The stomach mucosa is composed of an epithelial layer organized into glands. Four different regions are differentiated from bottom to top: base, neck, isthmus and pit. The corpus epithelium presents long glands with short pits containing mucus secreting pit and neck cells, acid secreting parietal cells, hormone secreting endocrine cells and digestive enzyme secreting chief cells (Fig. 1B) [7]. Antral glands are shorter, and have a relatively larger pit region. They also contain mucus secreting pit and neck cells as well as endocrine cells, but fewer basally located chief cells and no parietal cells [8]. The isthmus region contains proliferating cells and has long been considered the home of gastric epithelial stem cells [9]. As suggested by their names, mucus pit cells are found exclusively in the pit region and mucus neck cells in the neck region, whereas chief cells are located at the base region only. Hormone producing endocrine and acid secreting parietal cells scatter throughout the whole gland. Gastric organoids Adult stem cells and pluripotent stem cells allow the establishment of gastric organoids Gastric organoids may be initiated from stomach tissue derived AdSCs as well as PSCs. The main difference between the systems is the presence of mesenchymal cells within the PSC-derived organoid culture. AdCSs can only generate the specific cells from the tissue-of-origin, whereas PSCs have by nature the ability to differentiate into any cell types. Therefore, PSC-derived organoids require a stepwise differentiation protocol that guides PSC differentiation into the target tissue identity, while AdSC-derived gastric organoids from the start only require a single growth factor-enriched medium. Consequently, the time to differentiate PSCs into organoids takes ~30–60 days, while AdSC-derived organoids are established within 7–14 days. The first murine adult stem cell derived stomach organoid culture was established from antrum glands containing Lgr5+ stem cells. The protocol was developed on the basis of the intestinal organoid culture system by the addition of fibroblast growth factor 10 (FGF10) and the hormone gastrin (Fig. 2A) [14]. In addition, markers of chief cells (pepsinogen C (PGC)) and mucus neck cells (MUC6) could be observed. Reduction of the WNT concentration resulted in the generation of the differentiated lineages of mucous pit and endocrine cells, while parietal cells were not observed [14]. The same conditions were later used for murine corpus organoids originating from Troy+ stem cells (Fig. 2B) [19]. These organoids expressed markers of chief cells and mucus neck cells. Upon the withdrawal of WNT, Noggin and FGF10 differentiated pit cells could be observed, but no endocrine or parietal cells. Taken together, first data indicate the usefulness of patient derived organoids in therapy prediction. In our experience, cancer organoids can be established from biopsies within 2–3 weeks for ~50% of patients to an extend that allows to test responses to standard chemotherapeutics. This is similar to the time frame of the routine work (i.e., CT and laparoscopic staging, intravenous port implantation) that happens before starting the neoadjuvant chemotherapy. Patients in a palliative setting normally receive first line of chemotherapy according to established protocols, before further personalized approaches are applied. This usually gives enough time to expand cultures to perform drug screens, i.e., in the form of a high-throughput drug screen as established by Du et al., before suggestions for second/third line treatment regimens are needed [117]. In our view, it will therefore be possible in the future to integrate PDOs into the clinical decision-making process. Of note, Yan et al. reported a heterogeneous drug response between PDOs from different tumor regions of one tumor [84]. Further co-clinical and first prospective studies in larger patient cohorts are therefore needed to clearly define the role of PDOs in different therapy settings (i.e., neoadjuvant/ palliative). In addition, for novel immunotherapeutic approaches, co-culture systems with immune cells also need to be validated concerning in vitro functionality of tumorimmune cell interaction [118]. First promising data hint toward the possibility for functional testing of e.g., checkpoint inhibition therapies using immune cell-organoid cocultures (Fig. 5C) [119]. Finally, for further interest we would like to direct the readers to other excellent reviews on different aspects of the topic of organoids [40, 100, 120–130]. The generation of tumor organoid libraries from other entities besides gastric cancer is reviewed in several articles [131–136]. |