Research Focus |
|
癌症患者来源的类器官:提高精准医疗的疗效 2024-04-12 17:52:55 浏览次数:946 | |
| 癌症患者来源的类器官:提高精准医疗的疗效 来源:仪方生物 www.yeslab.com 随着发现癌症(PCa)的临床和分子景观的进展,在临床上实施精确的医学指导治疗测试已成为优先事项。患者来源的类器官(PDO)是三维(3D)组织培养物,有望通过对PCa建模来预测具有可靠疗效的治疗反应,从而在精准医学和临床试验中验证临床前药物测试。我们评估了3D培养和PDO用于克隆异质性建模和筛选有效靶向治疗的进展,重点是生成PDO的技术进展。最近的创新包括在原始研究和/或临床试验中的相关研究中使用PDO来检查药物在前列腺癌肿瘤微环境(TME)中的作用。利用各种细胞外基质和单细胞测定法产生和长期繁殖PDO也有了显著的改善。单细胞来源的PDO可以忠实地再现原始肿瘤并反映其异质性特征。虽然大多数PDO在精准医学中的使用都涉及转移患者的组织,这是可以理解的,但我们设想,从局部前列腺癌中产生PDO,以及将TME的细胞纳入组织模型,将发挥PDO在预测药物临床效益方面的巨大潜力。我们的结论是,单细胞衍生的PDO重申了原始肿瘤的分子特征,并代表了一个可靠的临床前PCa模型,以了解单个肿瘤并设计量身定制的靶向治疗。在世界各地的男性中诊断,仅落后于肺癌癌症。2020年,全球PCa新增病例超过1414259例,死亡375304例[1]。值得注意的是,尽管在过去十年中,癌症死亡率加速下降,但PCa死亡率的下降速度停止了[1]。 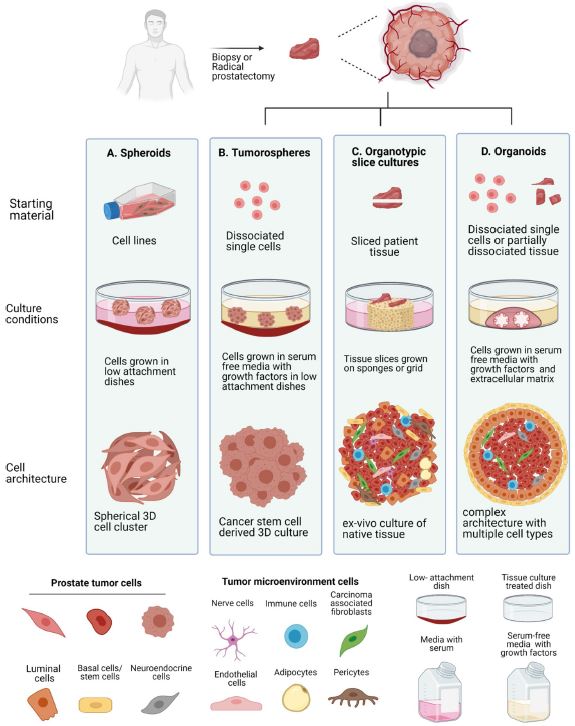 雄激素受体信号传导在前列腺癌的生长和存活中起着积极作用,使雄激素剥夺疗法(ADT)的药物阉割成为前列腺癌标准护理的支柱。然而,许多接受ADT治疗的患者发展为去势耐受性前列腺癌(CRPC),这是通过尽管进行了去势,但前列腺特异性抗原水平升高来诊断的。目前批准的CRPC治疗方法包括激素治疗、化疗、免疫疗法、放射性核素治疗和基于生物标志物的靶向治疗,如PARP抑制剂。尽管有几种新的治疗选择,但转移性CRPC(mCRPC)仍然是一种致命疾病,从进展时起存活率低于2-3年[2]在精准肿瘤学时代,PCa的有效治疗依赖于预测性分子特征来设计最佳治疗序列和组合策略[3]。最近的研究已经确定了基因组/转录组学特征,这些特征捕捉了局部前列腺癌(如Ets融合)和CRPC相关基因改变(AR、TP53、PTEN、BRCA 1/2)的早期发病事件[4]。然而,由于对肿瘤组织进行分子分析的途径有限,缺乏可靠的方法来捕捉不同疾病状态下的肿瘤异质性,以及临床前模型不完善,这些分子生物标志物在临床上用于治疗选择的进展受到了阻碍[3]。在前列腺癌诊所实施精准医学的关键是利用临床前模型,该模型可以1)忠实地概括患者肿瘤的细胞、结构和分子特征,2)实现药物测试和/或高通量筛选(HTS),以及3)允许及时将治疗建议转译到诊所。使用患者来源的类器官(PDO)可以为符合多种治疗条件的患者提供一种有效的策略来确定最佳药物选择。在这篇综述中,我们总结了前列腺PDO的过去、现在和未来,概述了PCa-PDO如何用于改善治疗决策过程,并指导转移性疾病的顺序或组合疗法的选择。 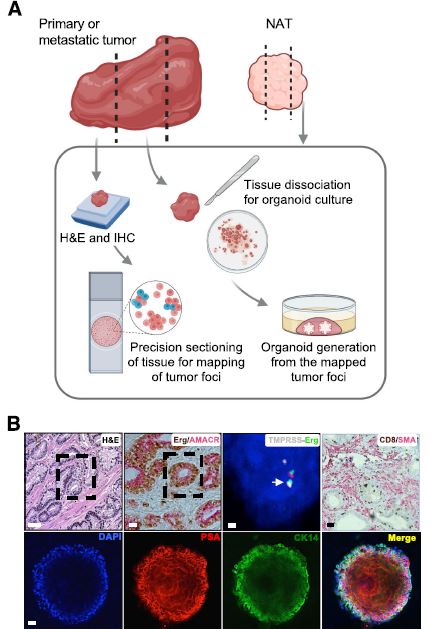 各种临床前模型已被用于推进前列腺癌的研究。大多数研究依赖于使用在二维(2D)培养物中生长或移植在免疫功能受损动物中的永生细胞系。尽管这些前列腺癌细胞系易于获得且使用简单,但只有少数存在,而且随着其长期培养,它们远不是原发性疾病的真正代表。此外,细胞系未能捕捉到肿瘤异质性的各个方面。患者来源的异种移植物(PDX)是一种更优越的前列腺癌临床前模型,因为它们保留了原始肿瘤的表型特征[5]。虽然PDX具有包括微环境的特定优势,但它们仍然存在于免疫功能受损的宿主鼠环境的限制范围内,并且仅提供低通量的治疗评估潜力。此外,用于药物筛选的PDX的成功生成需要几个月的时间才能完成[5,6]。此外,PDX和前列腺癌细胞系经常来源于侵袭性转移性疾病,因此缺乏用于研究原发性局部前列腺癌的临床前模型。 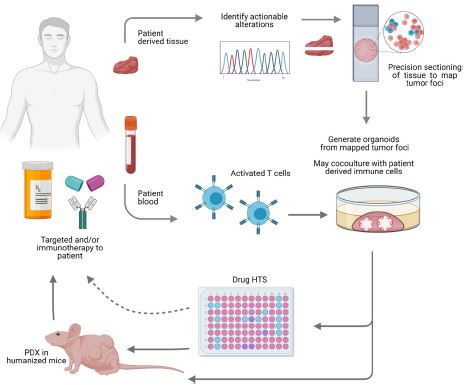 在过去的十年里,前列腺癌研究取得了重大进展。随着人们对前列腺癌的起源和分子景观的了解不断加深,以精准医学为基础的方法治疗晚期前列腺癌的趋势令人鼓舞。PDO能够更好地理解PCa起始和进展的复杂性。随着使用PDO的药物HTS的进展,这些进展使PDO成为主要的临床前模型。它们在预测治疗反应方面的广泛应用仍存在一些障碍。关键的限制是在已建立的PDO培养物中缺乏基质、免疫和内皮细胞成分。为了在前列腺癌中实施有利于精准医学的PDO,需要一种跨学科的方法。该方法包括使用3D组织工程、大规模基因组和形态学分析、在人源化小鼠中植入PDO,以及用液体活检进行纵向评估,以评估宿主微环境中的靶向治疗效果[40]。我们设想,开发新的方法,允许共同研究PDO中的肿瘤细胞和TME,将进一步推进PDO在临床医学中的应用。 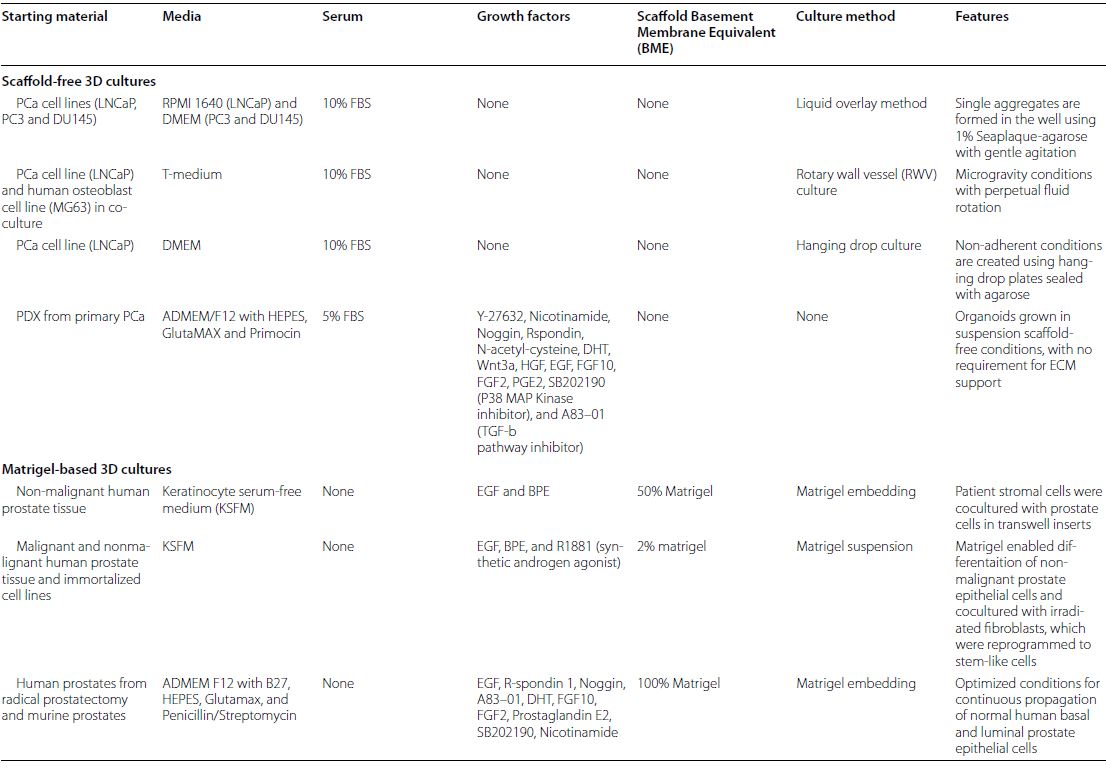 Patient derived organoids in prostate cancer: improving therapeutic efficacy in precision medicine With advances in the discovery of the clinical and molecular landscapes of prostate cancer (PCa), implementation of precision medicine-guided therapeutic testing in the clinic has become a priority. Patient derived organoids (PDOs) are three-dimensional (3D) tissue cultures that promise to enable the validation of preclinical drug testing in precision medicine and coclinical trials by modeling PCa for predicting therapeutic responses with a reliable efficacy. We evaluate the advances in 3D culture and PDO use to model clonal heterogeneity and screen for effective targeted therapies, with a focus on the technological advances in generating PDOs. Recent innovations include the utilization of PDOs both in original research and/or correlative studies in clinical trials to examine drug effects within the PCa tumor microenvironment (TME). There has also been a significant improvement with the utilization of various extracellular matrices and single cell assays for the generation and long-term propagation of PDOs. Single cell derived PDOs could faithfully recapitulate the original tumor and reflect the heterogeneity features. While most PDO use for precision medicine understandably involved tissues derived from metastatic patients, we envision that the generation of PDOs from localized PCa along with the incorporation of cells of the TME in tissue models would fulfill the great potential of PDOs in predicting drug clinical benefits. We conclude that single cell derived PDOs reiterate the molecular features of the original tumor and represent a reliable pre-clinical PCa model to understand individual tumors and design tailored targeted therapies. diagnosed in men worldwide, only behind lung cancer. In 2020, over 1,414,259 new PCa cases and 375,304 deaths were estimated for PCa worldwide [1]. Notably, while the past decade has seen an accelerated decline in the death rate of lung cancer, reduction rate halted for PCa [1]. Androgen receptor signaling plays an active role in the growth and survival of PCa, making medical castration with androgen deprivation therapy (ADT) a mainstay in PCa standard of care. However, many ADT-treated patients develop castration-resistant PCa (CRPC), which is diagnosed by rising levels of prostate-specific antigen despite castration. Current approved treatments for CRPC include hormonal therapy, chemotherapy, immunotherapy, radionuclide therapy, and biomarker based targeted therapies such as PARP inhibitors. Despite several new treatment options, metastatic CRPC (mCRPC) remains a lethal disease with a survival rate below 2–3 years from the time of progression [2] Effective treatment for PCa in the era of precision oncology relies on predictive molecular signatures to design optimal treatment sequence and combination strategies [3]. Recent studies have identified genomic/ transcriptomic signatures capturing the early pathogenetic events in localized PCa (e.g., Ets fusions) and CRPC-associated gene alterations (AR, TP53, PTEN, BRCA 1/2) [4]. However, progress on the use of such molecular-biomarkers for treatment selection in the clinic has been hampered by the limited access to tumor tissue for molecular profiling, the lack of reliable approaches to capture tumor heterogeneity at different disease states, and the imperfect preclinical models [3]. A key for implementing precision medicine in the PCa clinic is to utilize preclinical models that can 1) faithfully recapitulate the cellular, structural, and molecular features of the patient’s tumor, 2) enable drug testing and/ or high throughput screening (HTS), and 3) allow the translation of therapy recommendations to the clinic in a timely manner. The use of patient derived organoids (PDOs) could provide an effective strategy to identify optimal drug choices for patients eligible for multiple treatment. In this review, we summarize the past, present, and future of prostate PDOs, outlining how PCa PDOs can be used to improve the therapeutic decisionmaking process and guide the selection of sequential or combinatorial therapies for the metastatic disease. Various preclinical models have been used to advance PCa research. Most studies relied on using immortalized cell lines grown in two-dimensional (2D) cultures or engrafted in immunocompromised animals. Despite having these PCa cell lines readily available and simple to use, only a handful exists and with their prolonged culture, they are far from being true representatives of the primary disease. In addition, cell lines fail to capture the various aspects of tumor heterogeneity. Patient-derived xenografts (PDX) are a more superior preclinical models of PCa, since they retain the phenotypic characteristics of the original tumor [5]. While PDXs have the specific advantage of including a microenvironment, they still exist within the limitation of immunocompromised host murine environment and only offer low-throughput therapeutic assessment potential. Furthermore, successful generation of PDXs for drug screening takes several months to accomplish [5, 6]. Moreover, both PDXs and PCa cell lines are frequently derived from the aggressive metastatic disease and therefore there is a paucity of preclinical models for studying primary locoregional PCa. The past decade has seen significant advances in PCa research. With the increased understanding of the origin and the molecular landscape of PCa, there has been an encouraging trend of precision medicine-based approach to treat advanced PCa. PDOs enabled a better comprehension of the complexity of PCa initiation and progression. With the advances in drug HTS employing PDOs, these advances have catapulted PDOs as a mainstay preclinical model. There still remains some barriers for their widespread use to predict treatment responses. The key limitation is the lack of stromal, immune, and endothelial cell components in established PDO cultures. To implement PDOs benefiting precision medicine in PCa, an interdisciplinary approach is required. This approach involves the use of 3D tissue engineering, large scale genomic and morphological profiling, engraftment of PDOs in humanized mice, and longitudinal assessment with liquid biopsies to evaluate targeted therapy effects in the host microenvironment [40]. We envision that the development of novel approaches allowing the co-investigation of tumor cells and TME in PDOs will further advance the applications of PDOs in clinical medicine. |
