Research Focus |
|
奈拉替尼可作为单一疗法或与曲妥珠单抗联合治疗HER2-低乳腺癌症细胞和类器官模型 2024-04-17 20:13:31 浏览次数:902 | |
| 奈拉替尼可作为单一疗法或与曲妥珠单抗联合治疗HER2-低乳腺癌症细胞和类器官模型 来源:仪方生物 www.yeslab.com 先前的研究表明,HER2低乳腺癌患者不能从曲妥珠单抗治疗中获益,尽管原因尚不清楚。我们使用生物化学方法和细胞活力测定,研究了曲妥珠单抗单药治疗及其与不同HER2靶向治疗联合治疗在一组乳腺癌症细胞系和患者来源的类器官(PDO)中的效果。与敏感的HER2过表达(IHC3 + ) 癌症细胞,增加曲妥珠单抗剂量不能达到MDA-MB-361的IC50(IHC 2 + 鱼 + ) 和MDA-MB-453(IHC 2 + FISH)细胞,其显示出对曲妥珠单抗的中间应答。曲妥珠单抗治疗诱导HER配体释放的上调,导致这些细胞中HER受体的激活,这可能是其对曲妥珠珠单抗不敏感的原因。添加双ADAM10/17抑制剂以抑制HER配体的脱落,并与曲妥珠单抗联合使用,仅显示出HER2低乳腺癌症细胞和PDO的细胞活力适度降低。然而,panHER抑制剂奈拉替尼是治疗HER2低乳腺癌症细胞和PDO的有效单药疗法,并在与曲妥珠单抗联合使用时显示出叠加效应。 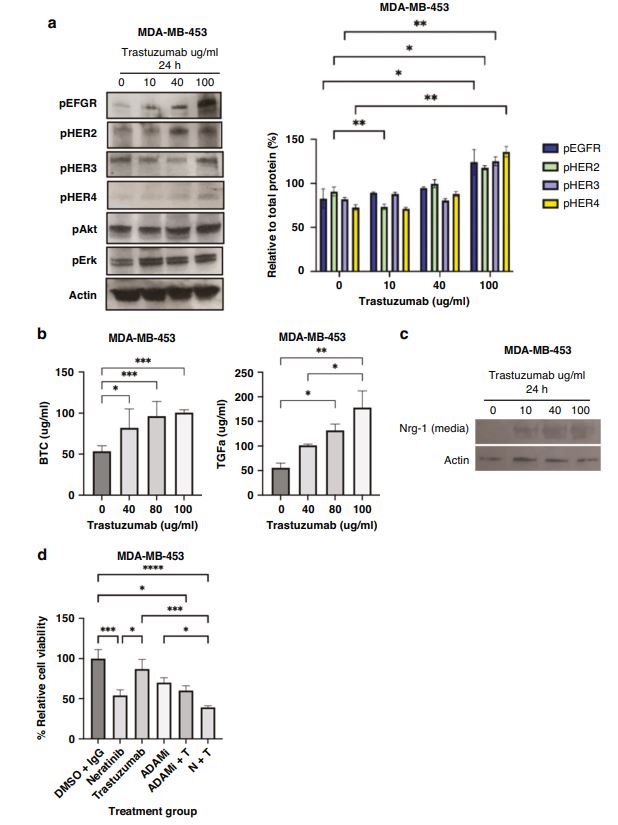 这项研究表明,奈拉替尼与曲妥珠单抗联合治疗HER2低乳腺癌可能有效,尽管需要在更大的PDO小组和未来的临床研究中进行进一步验证。 在精准医疗时代,人类表皮生长因子受体2(HER2)是癌症最重要的预测和预后生物标志物[1]。根据ASCO(美国临床肿瘤学会)[2]的指导,通过免疫组织化学(IHC)和/或原位杂交(ISH)评估HER2状态对于选择合适的患者接受曲妥珠单抗和/或其他抗HER2治疗在临床上至关重要。通常建议患有HER2阳性肿瘤(定义为IHC3+和/或HER2拷贝数≥6,如果FISH/CEP17比值<2,和/或HER2拷贝数≤4,如果FISH/CEP17比率≥2)的患者使用曲妥珠单抗和其他抗HER2疗法进行治疗。先前显示HER2阳性(IHC3 + ) 与IHC2+或更低的肿瘤相比,肿瘤更有可能对曲妥珠单抗和化疗产生反应( < 2) 得分[3]。然而,由于使用不同的抗体、染色平台和阳性截断值的变化,IHC或FISH在确定HER2状态方面存在一些差异[4]。随着最近2018年ASCO/CAP HER2测试指南的引入,这种差异有所改善。由于先前HER2状态分类的模糊性,在早期研究中发现一些HER2低肿瘤患者受益于抗HER2治疗。Paik等人(2008)证明,一些肿瘤患者最初被确定为HER2阳性,但后来通过IHC/FISH评分被定义为HER2阴性,也通过含有曲妥珠单抗的辅助治疗改善了生存结果[5]。此外,最近的DESTINY-Breast04试验表明,与医生选择的HER2低转移性癌症患者化疗相比,曲妥珠单抗deruxtecan可显著延长患者的无进展期和总生存期[6]。因此,鉴于最初的阈值是为曲妥珠单抗治疗设定的[7,8],而不是为其他抗HER2治疗或其组合设定的,因此,除了曲妥珠mab deruxtecan外,HER2低肿瘤可能受益于其他FDA批准的抗HER2疗法。 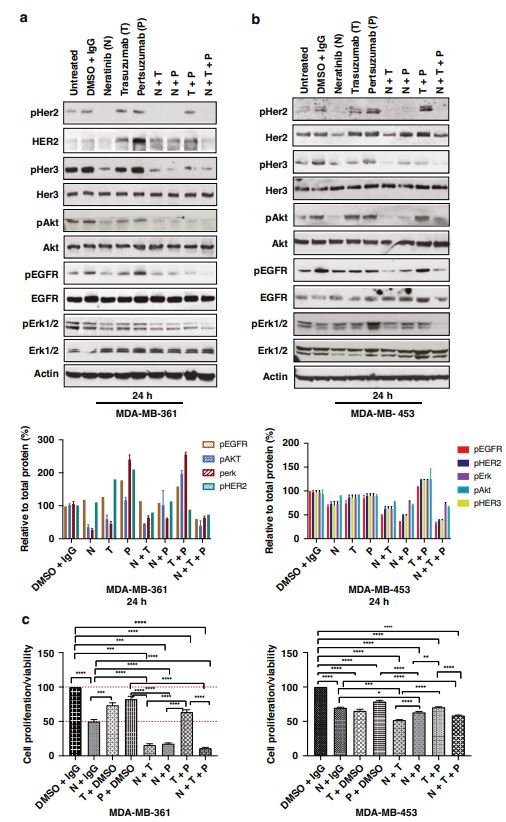 在22个已鉴定的人类ADAM(一种去整合素和金属蛋白酶)家族中,ADAM 10和17是研究最多的成员,它们通过HER配体和/或受体的脱落来调节HER受体信号传导[20]。我们之前报道,ADAM10和ADAM17金属蛋白酶通过HER2阳性乳腺癌症细胞中HER配体的脱落和HER受体的激活介导对曲妥珠单抗治疗的耐药性[21,22]。然而,尚未研究ADAM10和17蛋白酶在曲妥珠单抗治疗HER2-低乳腺癌症细胞中的作用。 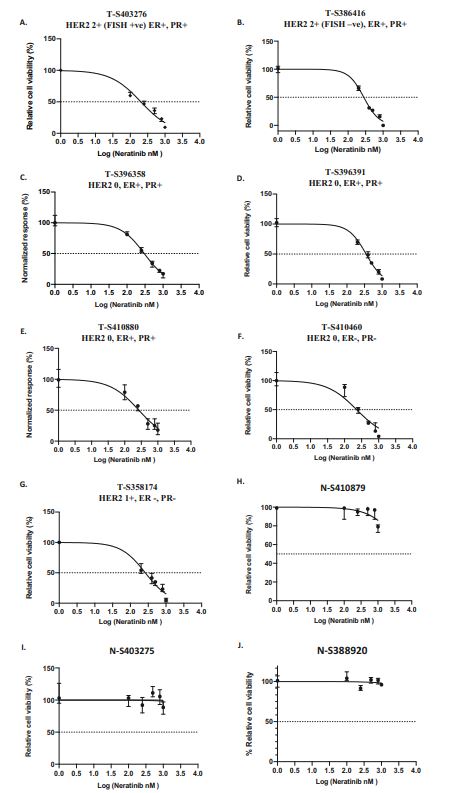 我们研究的目的是研究HER受体激活是否发生在由ADAM10/17配体释放介导的曲妥珠单抗治疗对HER2低乳腺癌症细胞的反应中。我们将评估通过a)使用ADAM10/17抑制剂靶向ADAM蛋白酶或b)使用奈拉替尼和/或帕妥珠单抗联合曲妥珠单抗抑制HER受体激活和二聚化来提高HER2低乳腺癌症细胞和患者来源的类器官(PDOs)[23]的治疗效果的可能性。 曲妥珠单抗的敏感性取决于HER2状态,低HER2乳腺癌症细胞对曲妥珠珠单抗不敏感 我们通过IHC和FISH确定了一组不同的癌症细胞系的HER2状态,这揭示了细胞系之间相当大的异质性(图S1A,S1B)。SK-BR-3和BT474都显示出强的IHC染色3+和增加的HER2基因扩增,而BT20和MCF-7细胞显示出弱到检测不到的IHC着色(1+和0)和正常的FISH状态。其他四种细胞系(MDA-MB-361、HCC-1569、ZR-75-1、MDA-MB-453)具有中间IHC 2+染色,并且在这些细胞系中MDA-MB.361和HCC-1569具有≥2的FISH比率,因此被认为是HER2对FISH扩增呈阳性。有趣的是,MDA-MB-453显示出比ZR-75-1弱的IHC染色,但FISH/CEP17比率略高(图S1A,S1B)。 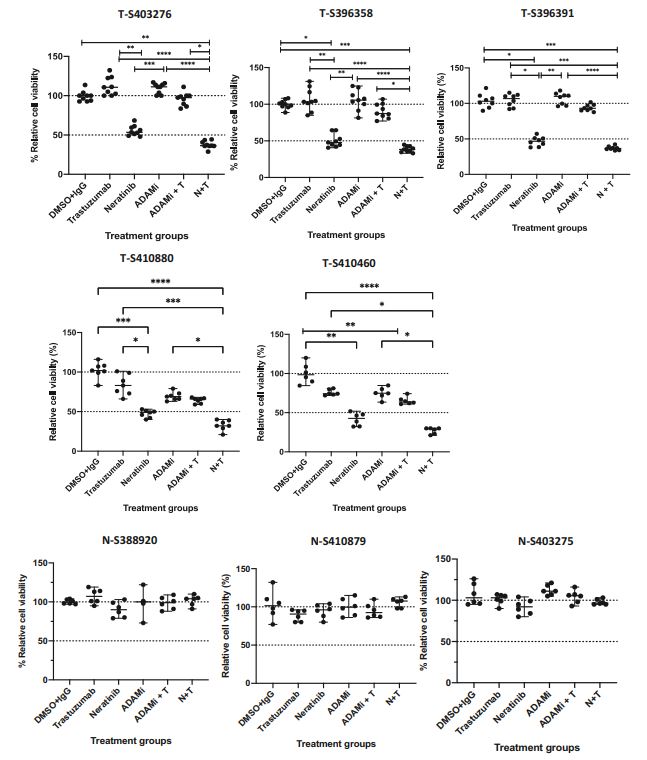 通过细胞活力评估抗HER2治疗对IHC 2+乳腺癌症细胞系的生长抑制作用,并与IHC 3中的抗HER2处理进行比较 + SK-BR-3和BT474细胞(图S1C)。必要时使用以可忽略的水平表达HER2的MCF-7细胞作为阴性对照。增加曲妥珠单抗剂量对MCF7(IHC 0)和BT20细胞(IHC 1)的细胞活力没有影响 + ) (图S1C)。另一方面,与IHC3相比,曲妥珠单抗导致四种HER2 IHC2+乳腺癌症细胞系的细胞活力轻度至中度降低 + 对曲妥珠单抗敏感的BT474和SKBR3细胞(IC50 < 10 μg/ml)(图S1C)。由于对曲妥珠单抗、MDA-MB-361(IHC HER2 2 + /钓鱼HER2 + ) 和MDA-MB-453(IHC HER2 2 + /选择FISH HER2-)乳腺癌症细胞系进行后续研究。 总之,我们已经表明,奈拉替尼单药治疗和曲妥珠单抗联合治疗对一小部分HER2-低乳腺癌症细胞和PDO有效。需要进一步的研究来评估奈拉替尼与曲妥珠单抗联合化疗或抗体偶联物(如TDM1或曲妥珠珠单抗-德鲁克斯替康)在这些HER2低肿瘤中的作用。还需要进一步的研究来研究其他基于奈拉替尼的组合,如在HER2-低ER-阳性乳腺癌症细胞和PDO中与激素治疗和/或CDK4/6抑制剂组合的奈拉替宁,以及在HER2-弱/TNBC细胞和PDOs中与抗PDL1/PD1组合的奈拉替尼。这将有助于设计临床试验,在未来将奈拉替尼整合到HER2低乳腺癌的治疗途径中。 Neratinib could be effective as monotherapy or in combination with trastuzumab in HER2-low breast cancer cells and organoid models Background Previous studies have suggested that patients with HER2-low breast cancers do not benefit from trastuzumab treatment although the reasons remain unclear. Methods We investigated the effect of trastuzumab monotherapy and its combination with different HER2 targeting treatments in a panel of breast cancer cell lines and patient-derived organoids (PDOs) using biochemical methods and cell viability assays. Results Compared to sensitive HER2 over-expressing (IHC3 + ) breast cancer cells, increasing doses of trastuzumab could not achieve IC50 in MDA-MB-361 (IHC 2 + FISH + ) and MDA-MB-453 (IHC 2 + FISH-) cells which showed an intermediate response to trastuzumab. Trastuzumab treatment induced upregulation of HER ligand release, resulting in the activation of HER receptors in these cells, which could account for their trastuzumab insensitivity. Adding a dual ADAM10/17 inhibitor to inhibit the shedding of HER ligands in combination with trastuzumab only showed a modest decrease in the cell viability of HER2-low breast cancer cells and PDOs. However, the panHER inhibitor neratinib was an effective monotherapy in HER2-low breast cancer cells and PDOs, and showed additive effects when combined with trastuzumab. Conclusion This study demonstrates that neratinib in combination with trastuzumab may be effective in a subset of HER2-low breast cancers although further validation is required in a larger panel of PDOs and in future clinical studies. In the era of precision medicine, Human Epidermal Growth Factor Receptor 2 (HER2) is the most important predictive and prognostic biomarker in breast cancer [1]. The assessment of HER2 status by immunohistochemistry (IHC) and/or in situ hybridization (ISH) is clinically essential to select appropriate patients for trastuzumab and/or other anti-HER2 treatments as guided by ASCO (American Society of Clinical Oncology) [2]. Patients with HER2 positive tumours (defined as IHC 3+ and/or HER2 copy number ≥ 6 if FISH/CEP17 ratio <2 and/or HER2 copy number ≥ 4 if FISH/CEP17 ratio ≥ 2) are usually recommended for treatment with trastuzumab and other anti-HER2 therapies. It was previously shown that HER2-positive (IHC 3 + ) tumours were more likely to respond to trastuzumab and chemotherapy than those tumours with IHC 2+ or lower ( < 2) scores [3]. However, there are some discrepancies in determining HER2 status by either IHC or FISH due to the use of different antibodies, staining platforms, and variation in cut off values for positivity [4]. The discrepancies have since improved with the introduction of the recent 2018 ASCO/CAP HER2 testing guideline. Due to this ambiguity in the classification of HER2 status previously, some patients with HER2-low tumours were found to benefit from anti-HER2 treatments in earlier studies. Paik et al. (2008) demonstrated that some patients with tumours originally identified as HER2 positive but later defined as HER2 negative by IHC/FISH score, also had improved survival outcomes from adjuvant trastuzumab containing treatment [5]. Moreover, the recent DESTINY-Breast04 trial showed that trastuzumab deruxtecan resulted in significantly longer progression-free and overall survival compared to the physicians choice of chemotherapy in patients with HER2-low metastatic breast cancer [6]. Therefore, in view of the original threshold was set for trastuzumab treatment [7, 8] but not for the other anti-HER2 treatments or their combination, it is likely that HER2-low tumours may benefit from other FDA approved anti-HER2 treatments in addition to trastuzumab deruxtecan. Among the 22 identified human ADAM (a disintegrin and metalloproteinase) family, ADAM 10 and 17 are the most studied members, which regulate HER receptor signalling through the shedding of HER ligands and/or receptors [20]. We previously reported that ADAM10 and ADAM17 metalloproteases mediate resistance to trastuzumab treatment via shedding of HER ligands and the activation of HER receptors in HER2-positive breast cancer cells [21, 22]. However, the roles of ADAM10 and 17 proteases were not investigated in relation to trastuzumab treatment in HER2-low breast cancer cells. The objective of our study was to investigate whether HER receptor activation occurs in response to trastuzumab treatment mediated by ADAM10/17 ligand-release in HER2-low breast cancer cells. We will assess the possibility of improving treatment efficacy in HER2-low breast cancer cells and patient-derived organoids (PDOs) [23] by either a) targeting ADAM proteases using an ADAM10/17 inhibitor or b) inhibiting HER receptor activation and dimerization using neratinib and/or pertuzumab in combination with trastuzumab. Trastuzumab sensitivity is dependent on HER2 status and HER2-low breast cancer cells are not sensitive to trastuzumab We determined the HER2 status of a panel of different breast cancer cell lines by IHC and FISH, which revealed considerable heterogeneity amongst cell lines (Fig. S1A, S1B). Both SK-BR-3 and BT474 showed strong IHC staining 3+ and increased HER2 gene amplification, whereas BT20 and MCF-7 cells showed weak to undetectable IHC staining (1+ and 0) and a normal FISH status. The other four cell lines (MDA-MB-361, HCC-1569, ZR-75-1, MDA-MB-453) had an intermediate IHC 2+ staining and among these cell lines MDA-MB-361 and HCC1569 had a FISH ratio of ≥ 2 and therefore were considered as HER2 positive for FISH amplification. Interestingly, MDA-MB-453 showed weaker IHC staining than ZR-75-1 but slightly higher FISH/CEP17 ratio (Fig. S1A, S1B). The growth inhibitory effects of anti-HER2 treatment in IHC 2+ breast cancer cell lines were assessed via cell viability and compared to anti-HER2 treatments in IHC 3 + SK-BR-3 and BT474 cells (Fig. S1C). The MCF-7 cells that express HER2 at a negligible level were used as a negative control when necessary. Increased trastuzumab dosages showed no effect on cell viability in MCF7 (IHC 0) and BT20 cells (IHC 1 + ) (Fig. S1C). Trastuzumab, on the other hand, resulted in a slight to moderate reduction in cell viability in four HER2 IHC2+ breast cancer cell lines compared to IHC 3 + BT474 and SKBR3 cells that were sensitive to trastuzumab (IC50 < 10 μg/ml) (Figure S1C). Due to intermediate response to trastuzumab, MDA-MB-361 (IHC HER2 2 + /FISH HER2 + ) and MDA-MB-453 (IHC HER2 2 + /FISH HER2-) breast cancer cell lines were chosen for our subsequent studies. In summary, we have shown that neratinib monotherapy and in combination with trastuzumab was effective in a small subset of HER2-low breast cancer cells and PDOs. Further studies are required to assess the effects of neratinib with trastuzumab in combination with chemotherapy or antibody conjugates such as TDM1 or trastuzumab deruxtecan in these HER2-low tumours. Further studies are also required to investigate other neratinib based combinations such as neratinib in combination with hormone treatments and/or CDK4/6 inhibitors in HER2-low ER-positive breast cancer cells and PDOs, as well as neratinib in combination with anti-PDL1/PD1 in HER2-low/TNBC cells and PDOs. This will help to design clinical trials to integrate neratinib in the treatment pathways of HER2-low breast cancers in the future. |
