Research Focus |
|
膀胱癌治疗的临床前模型研究 2024-04-25 08:42:45 浏览次数:2172 | |
| 膀胱癌治疗的临床前模型研究 来源:仪方生物 www.yeslab.com 目的 膀胱癌(BC)是一种高度异质性疾病,包括各种分子亚型和组织学变异的肿瘤。这种异质性代表了新疗法发展的主要挑战。临床前模型,密切模拟体内肿瘤和反映其多样性的生物学是必不可少的,以确定具有特定活性的治疗各种BC亚型。在本综述中,我们总结了过去24个月在这方面所作的努力和取得的进展。 最近的发现 近年来,一个主要的焦点放在开发病人衍生的BC模型上。病人来源的类器官(PDO)和病人来源的异种移植物(PDX)被证明广泛地再现了相应来源肿瘤的分子和组织病理学特征以及药物反应特征。因此,这些模型代表了药物开发和个性化医疗的有希望的工具。除PDX外,体内同基因/同种异体模型也越来越重要。由于这些模型是使用具有免疫活性的宿主生成的,因此除其他外,它们可用于开发新的免疫疗法,并评估免疫系统对药物反应和耐药性的影响。 总结 在过去的两年里,各种在体和体外模型密切再现了生物学和异质性的膀胱肿瘤的发展。 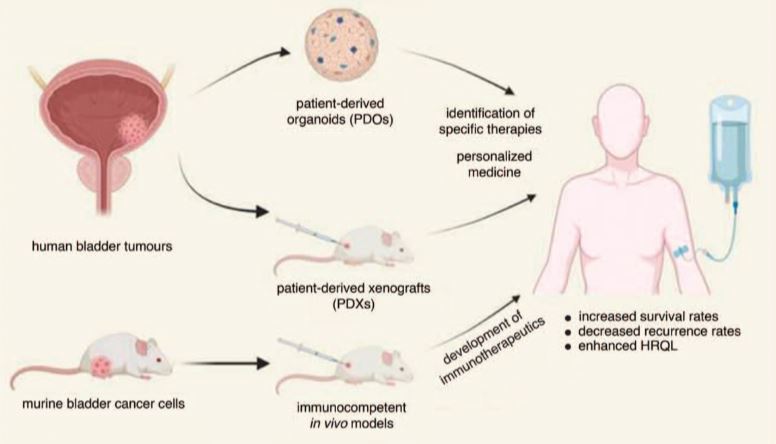 膀胱癌(BC)估计每年新增55万例,死亡20万例,是最常见的恶性肿瘤之一。尽管最近取得了一些突破,例如批准了泛成纤维细胞生长因子受体(FGFR)抑制剂厄达非尼和几种免疫检查点抑制剂(如pembrolizumab、nivolumab、avelumab),以及开发了抗体-药物偶联物(如enfortumab-vedotin),但晚期和转移性BC患者的预后仍然很差[2-4]。 膀胱肿瘤的高度异质性是新疗法发展的主要障碍。虽然约75%代表纯尿路上皮癌(UC),其余25%包括各种组织学变体,如鳞状细胞癌(SCC)、腺癌或肉瘤样癌(SaC)[5]。此外,大量研究表明,BC可细分为不同的分子亚型,对目前可用的疗法具有不同的敏感性[6,7]。 为了建立在各种BC亚型中具有特异活性的新的治疗方案,密切模拟体内肿瘤的临床前模型是必不可少的。在本综述中,我们总结了过去24个月在这方面所作的努力和取得的进展。鉴于在此期间发表的文献,我们将主要关注三维(3D)体外培养,以及患者来源的异种移植和同基因/同种异体体内模型(图1)。 体外模型 几十年来,药物开发的体外研究主要是利用成熟的商业化BC细胞系的2D培养进行的。尽管这些模型代表了强大的工具,但它们在影响其临床代表性方面具有相当大的局限性。一方面,经典的BC细胞系已经培养了很多年,导致遗传和表观遗传改变的积累,每一代都会出现这种改变[8,9]。另一方面,传统的2D模型无法再现不同细胞类型或癌细胞与肿瘤微环境之间的相互作用,这会显著影响药物反应[10]。 在过去的2年里,大多数研究小组专注于建立“类器官”,即由多种类型的细胞组成的3D结构[11]。Berndt Paetz等人开发了含有经典BC细胞系RT4、RT112、T24或CAL-29以及原代人类膀胱成纤维细胞和平滑肌细胞的类有机物。类器官紧密地模仿膀胱壁的倒转,其核心是由尿路上皮细胞包围的支持性细胞。在其他发现中,研究小组证明了类有机物和传统2D培养物在药物反应方面的显著差异[12▪]. Wei等人也证明了三维BC模型在药物开发方面的优越性,他们比较了BC细胞系和患者衍生类有机物(PDO)对顺铂、Bcl-2抑制剂venetoclax和MCL1抑制剂S63845的敏感性[13▪]. 为了建立个性化治疗方法的工具,Minoli等人建立了一个广泛的PDO集合,这些PDO来源于不同疾病阶段和级别的肿瘤。PDO被发现能够准确地代表其来源肿瘤的分子和组织病理学特征以及多克隆景观。通过测试PDO对标准护理治疗的敏感性,准确地再现了BC患者队列中观察到的治疗结果。因此,PDO被证明是一个有希望的工具,用于根据药物反应敏感性曲线对患者进行分层[14▪▪]. 基于这些发现,目前正在进行一项临床II期试验,旨在确定针对单个NMIBC患者的最有效滴注疗法[15▪▪]. 特别是在罕见的肿瘤实体,如SaC的情况下,缺乏临床前模型阻碍了特定药物的开发。为了解决这个问题,Garioni等人建立了一个长期的类器官SaC模型,以及代表传统UC的类器官模型。高通量药物筛选包括1567种化合物,从而识别出对UC或SaC以及两种疾病实体具有特定活性的候选药物[16▪]. 类器官模型也代表了新的免疫疗法的发展前景。Jiang等人产生了靶向跨膜蛋白B7H3的嵌合抗原受体(CAR)-T细胞,该蛋白在大多数癌症类型中高度表达。共培养的PDO进行CAR诱导裂解,从而确认特异性抗原识别和免疫激活[17▪]. 近年来,BC研究取得了显著进展,但仍存在明显挑战。尽管FGFR抑制剂、ICIs和ADCs等研究取得了进展,但晚期和转移性BC的预后仍然很差,高度异质性使治疗进展更加复杂。为了解决这一复杂性,人们已经加大了努力,特别关注于改进临床前模型,以更好地模拟患者肿瘤生物学。 虽然讨论的模型为膀胱癌研究提供了有希望的途径,但它们并非没有局限性。临床前模型,如类有机物和PDX,可能仍不能充分反映人类肿瘤的复杂性或长期治疗效果,可能导致药物反应的差异。此外,这些模型通常需要肿瘤取样的侵入性程序,限制了它们对纵向研究的适用性。此外,体内模型在重现人体免疫反应方面面临挑战,限制了其在免疫治疗发展中的应用。通过不断完善模型和治疗方法来解决这些局限性对于提高其预测准确性和临床实用性至关重要。 总之,先进的临床前模型和创新的治疗策略的融合为克服BC异质性和耐药机制带来的挑战带来了巨大的希望。利用3D类器官、PDX和同基因/同种异体模型有望增强药物发现、个性化治疗方法和免疫治疗发展。在这一方向上持续的跨学科努力对于将临床前发现转化为临床有效治疗,最终改善与这种高度流行疾病作斗争的患者的预后至关重要。 Preclinical models for bladder cancer therapy research Purpose of review Bladder cancer (BC) is a highly heterogenous disease comprising tumours of various molecular subtypes and histologic variants. This heterogeneity represents a major challenge for the development of novel therapeutics. Preclinical models that closely mimic in vivo tumours and reflect their diverse biology are indispensable for the identification of therapies with specific activity in various BC subtypes. In this review, we summarize efforts and progress made in this context during the last 24 months. Recent findings In recent years, one main focus was laid on the development of patient-derived BC models. Patient-derived organoids (PDOs) and patient-derived xenografts (PDXs) were demonstrated to widely recapitulate the molecular and histopathological characteristics, as well as the drug response profiles of the corresponding tumours of origin. These models, thus, represent promising tools for drug development and personalized medicine. Besides PDXs, syngenic/allogenic in vivo models are of growing importance. Since these models are generated using immunocompetent hosts, they can, amongst others, be used to develop novel immunotherapeutics and to evaluate the impact of the immune system on drug response and resistance. Summary In the past two years, various in vivo and in vitro models closely recapitulating the biology and heterogeneity of human bladder tumours were developed. With an estimated 550.000 new cases and 200.000 deaths annually, bladder cancer (BC) ranks amongst the most common malignancies [1]. Despite recent breakthroughs, such as the approval of the pan-fibroblast growth factor receptor (FGFR) inhibitor erdafitinib and several immune checkpoint inhibitors (e.g. pembrolizumab, nivolumab, avelumab), as well as the development of antibody-drug conjugates (e.g. enfortumab vedotin), patients suffering from advanced and metastatic BC still have a dire prognosis [2–4]. A major hindrance for the development of novel therapies is the high heterogeneity of bladder tumours. While approx. 75% represent pure urothelial carcinoma (UC), the remaining 25% comprise various histologic variants such as squamous cell carcinoma (SCC), adenocarcinoma, or sarcomatoid carcinoma (SaC) [5]. Moreover, numerous studies showed that BC can be subdivided into various molecular subtypes with different sensitivities to currently available therapies [6,7]. For the establishment of novel therapy schemes with specific activity in various BC subtypes, preclinical models that closely mimic in vivo tumours are indispensable. In this review, we summarize efforts and progress made in this context during the last 24 months. Given the literature published during this time period, we will mainly focus on 3-dimensional (3D) in vitro cultures, as well as on patient-derived xenograft and syngenic/allogenic in vivo models (Fig. 1). IN VITRO MODELS For several decades, in vitro studies for drug development were mainly conducted using 2D cultures of well established, commercially available BC cell lines. Even though these models represent powerful tools, they have considerable limitations affecting their clinical representativeness. On the one hand, classical BC cell lines have been cultured for many years, causing an accumulation of genetic and epigenetic alterations that arise with every passage [8,9]. One the other hand, traditional 2D models cannot recapitulate interactions between different cell types or cancer cells and the tumour microenvironment, that significantly influence drug response [10]. In the last 2 years, most research groups focused on the establishment of ‘organoids’ that are defined as 3D structures consisting of multiple types of cells [11]. Berndt-Paetz et al. developed organoids containing either of the classical BC cell lines RT4, RT112, T24 or CAL-29, as well as primary human bladder fibroblasts and smooth muscle cells. The organoids closely mimicked an inverse bladder wall, with a core of supportive cells surrounded by urothelial cells. Amongst other findings, the research group demonstrated significant differences in drug–response between organoids and conventional 2D cultures [12▪]. The superiority of 3-dimensional BC models in the context of drug development was also demonstrated by Wei et al., who compared the sensitivities of BC cell lines and of patient-derived organoids (PDOs) to cisplatin, the Bcl-2 inhibitor venetoclax and the MCL1 inhibitor S63845 [13▪]. In an attempt to establish tools for a personalized treatment approach, Minoli et al. established an extensive collection of PDOs deriving from tumours of various disease stages and grades. The PDOs were found to accurately represent the molecular and histopathological characteristics and multiclonal landscapes of their tumours of origin. Testing the sensitivities of the PDOs to standard of care therapies, treatment outcomes observed in BC patient cohorts were accurately recapitulated. PDOs were, thus, shown to be a promising tool for the stratification of patients according to drug response sensitivity profiles [14▪▪]. Based on these findings, a clinical phase II trial with the aim to identify the most effective instillation therapy for individual NMIBC patients is presently being conducted [15▪▪]. Especially in the case of rare tumour entities, such as SaC, the lack of preclinical models impedes the development of specific drugs. To address this issue, Garioni et al. established a long-term organoid-like SaC model, as well as organoids representing conventional UC. A high-throughput drug screen including 1567 chemical compounds led to the identification of drug candidates with specific activity against either UC or SaC, and against both disease entities [16▪]. Organoid models also represent promising tools for the development of novel immunotherapeutics. Jiang et al. generated chimeric antigen receptor (CAR)-T cells targeting the transmembrane protein B7H3, which is highly expressed in most cancer types. Co-cultured PDOs underwent CAR-induced lysis, thus confirming specific antigen recognition and immune activation [17▪]. The landscape of BC research is marked by significant advances in recent years, yet clear challenges persist. Despite advancements like FGFR inhibitors, ICIs and ADCs, the prognosis for advanced and metastatic BC remains bleak, with high heterogeneity further complicating therapeutic development. Efforts have intensified to address this complexity, with a particular focus on refining preclinical models to better mimic patient tumour biology. While the discussed models offer promising avenues for bladder cancer research, they are not without limitations. Preclinical models, such as organoids and PDXs, may still inadequately capture the complexity of human tumours or long-term treatment effects, potentially leading to discrepancies in drug responses. Moreover, these models often require invasive procedures for tumour sampling, limiting their applicability for longitudinal studies. Furthermore, in vivo models face challenges in recapitulating e.g., human immune responses, limiting their utility in immunotherapy development. Addressing these limitations through ongoing refinement of models and therapies is crucial to improving their predictive accuracy and clinical utility. Overall, the convergence of advanced preclinical models and innovative therapeutic strategies holds great promise for overcoming the challenges posed by BC heterogeneity and resistance mechanisms. Leveraging 3D organoids, PDXs, and syngenic/allogenic models promises to enhance drug discovery, personalized treatment approaches, and immunotherapy development. Continued interdisciplinary efforts in this direction are crucial for translating preclinical findings into clinically effective treatments, ultimately improving outcomes for patients battling this highly prevalent disease. |
